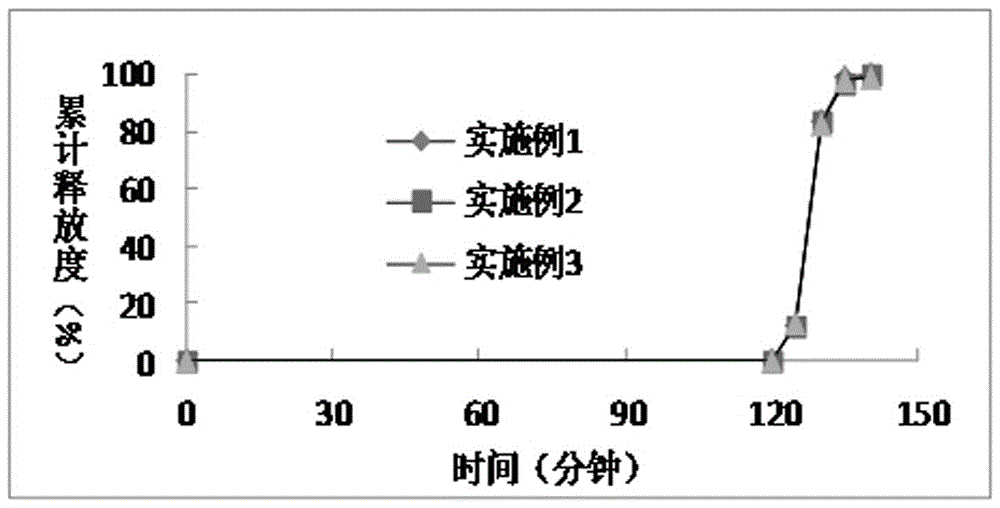
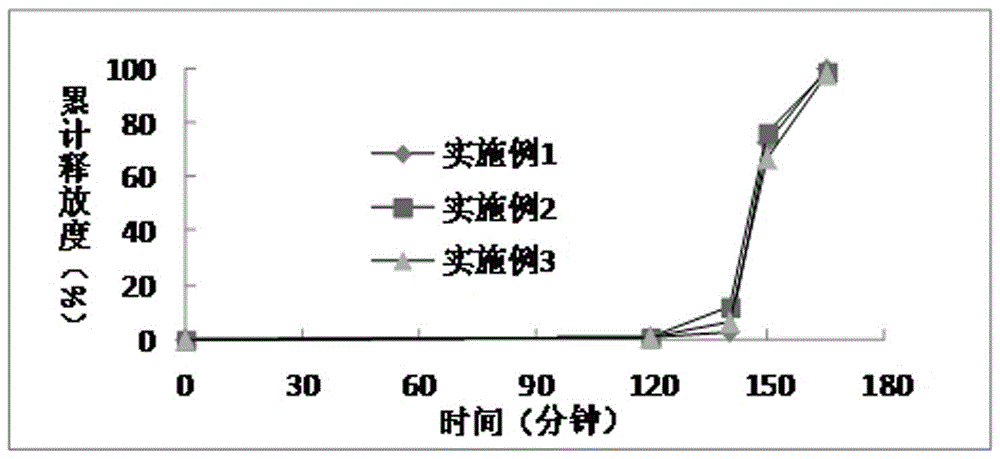
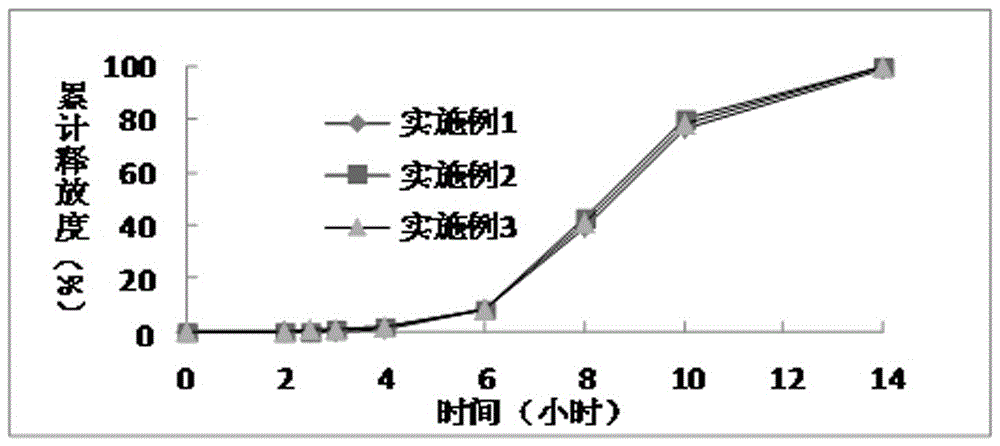
A kind of dimethyl fumarate enteric-coated pellets and preparation method thereof
A technology of dimethyl fumarate enteric and dimethyl fumarate, which is applied in the field of dimethyl fumarate enteric-coated pellets and the preparation thereof, and can solve the problem of easy sublimation loss of dimethyl fumarate and inability to obtain dimethyl fumarate. The release advantage of multi-unit enteric-coated preparations can not reduce the irritation of the gastrointestinal tract, and achieve the effect of reducing the toxic and side effects of the gastrointestinal tract, reducing the risk of in vitro dose dumping, and completely releasing the drug
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Composition of dimethyl fumarate enteric-coated pellets:
[0043] Ball core composition:
[0044] Raw material name 1000 dosage (g)
[0045] Dimethyl fumarate 120
[0046] Microcrystalline Cellulose 150
[0047] Sodium carboxymethyl starch 20
[0048] Hydroxypropyl Cellulose 13
[0049] 95% ethanol 103
[0050] Purified water 116
[0051] Composition of the inner enteric coating layer:
[0052] Raw material name dosage (g)
[0053] Udage L12.5 12.5
[0054] Triethyl citrate 1
[0056] 95% ethanol 170
[0057] Composition of the outer enteric coating layer:
[0058] Raw material name dosage (g)
[0059] Eudragit L30D-55 water dispersion 150
[0060] Triethyl citrate 5
[0061] Talc 12
[0062] Purified water 220
[0063] Note: The dry polymer content of Eudragit L30D-55 aqueous dispersion is 30%
[0064] Preparation Process:
[0065] (1) Accurately weigh dimethyl fumarate, microcrystalline cellulose, sodium carboxymethyl starch a...
Embodiment 2
[0075] Composition of dimethyl fumarate enteric-coated pellets:
[0076] Ball core composition:
[0077] Raw material name 1000 dosage (g)
[0078] Dimethyl fumarate 120
[0079] Microcrystalline Cellulose 100
[0080] Lactose 50
[0081] Croscarmellose Sodium 25
[0082] Hypromellose 14
[0083] 95% ethanol 103
[0084] Purified water 116
[0085] Composition of the inner enteric coating layer:
[0086] Raw material name dosage (g)
[0087] Udrake L100 15
[0088] Triethyl citrate 2
[0089] Talc 4
[0090] 95% ethanol 200
[0091] Composition of the outer enteric coating layer:
[0092] Raw material name dosage (g)
[0093] Cellulose acetate phthalate 50
[0094] Triethyl citrate 5
[0095] Talc powder 10
[0096] Purified water 220
[0097] Preparation Process:
[0098] (1) Accurately weigh dimethyl fumarate, microcrystalline cellulose, lactose, croscarmellose sodium and hydroxypropyl cellulose, and weigh 95% ethanol separately.
[0099] (2) Mix 95% etha...
Embodiment 3
[0108] Composition of dimethyl fumarate enteric-coated pellets:
[0109] Ball core composition:
[0110] Raw material name 1000 dosage (g)
[0111] Dimethyl fumarate 120
[0112] Microcrystalline Cellulose 150
[0113] Croscarmellose Sodium 20
[0114] Povidone K30 14
[0115] 95% ethanol 103
[0116] Purified water 116
[0117] Composition of the inner enteric coating layer:
[0118] Raw material name dosage (g)
[0119] Udrake L100 15
[0120] Triethyl citrate 5
[0121] Talc 5
[0122] 95% ethanol 200
[0123] Composition of the outer enteric coating layer:
[0124] Raw material name dosage (g)
[0125] Eudragit L30D-55 aqueous dispersion 170
[0126] Triethyl citrate 5
[0127] Talc 15
[0128]Purified water 220
[0129] Note: The dry polymer content of Eudragit L30D-55 aqueous dispersion is 30%
[0130] Preparation Process:
[0131] (1) Accurately weigh dimethyl fumarate, microcrystalline cellulose, croscarmellose sodium and hydroxypropyl cellulose, and...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com