Use of cardiac glycoside compound
A compound, cardiac glycoside technology, applied in the field of cardiac glycoside compounds, to achieve high efficiency, significant anti-tumor activity, and good quality of the extract
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] A. After 25.4 kg of calotropis gigantea stems are naturally air-dried and pulverized, by the calotropis gigantea sample: the mass / volume ratio (kg / L) of ethanol aqueous solution is about 1:2, with 50L volume fraction of ethanol aqueous solution of 95% Fully mixed with 25.4kg of horn melon samples, sealed, leached at room temperature for 3 times, each time for 7 days, filtered and combined the extracts, concentrated under reduced pressure until there was no alcohol smell to obtain the ethanol extract;
[0027] B. At room temperature, disperse the ethanol extract in water to make a suspension, according to the volume ratio of ethanol extract: petroleum ether = 1:3, 1:2, 1:1, repeat the ethanol extract with petroleum ether After extracting 3 times, and concentrating under reduced pressure to recover petroleum ether, petroleum ether extract (254.1 g) was obtained;
[0028] C. Filter the water liquid after petroleum ether extraction, separate the filtrate by D-101 macroporou...
Embodiment 2
[0033] After column chromatography, Fr.4-1 (3.2 g) in step E in Example 1 was eluted with an eluent with a volume ratio of chloroform:methanol=25:1, and the same components were collected by TLC thin layer chromatography , to obtain 7 parts Fr.4-1-1~Fr.4-1-7; Fr.4-1-4 (869.5mg) was subjected to silica gel column chromatography, followed by chloroform:methanol=40:1, chloroform : Acetone=6:2 volume ratio of the eluent for elution to finally obtain the cardiac glycoside compound calotropin (109.9mg). The study found that the structural difference between this compound and the target compound of the present invention is that there is no hydroxyl group on the 12-position carbon, while the 12-position carbon of the target compound of the present invention is substituted by a hydroxyl group. In the further in vitro anti-tumor activity test, it was found that the anti-IC50 values of the two compounds were significantly different (see Table 2).
Embodiment 3
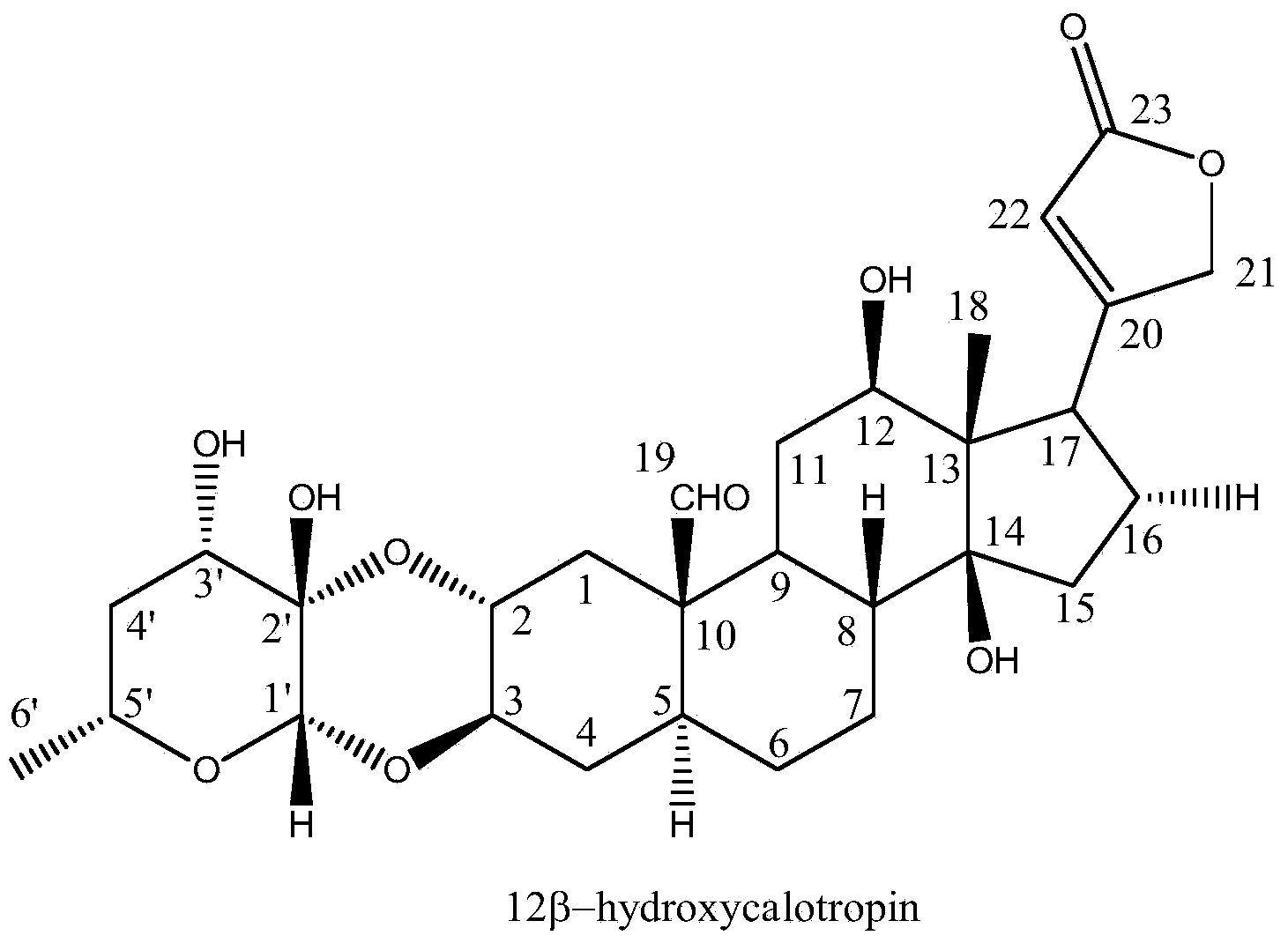
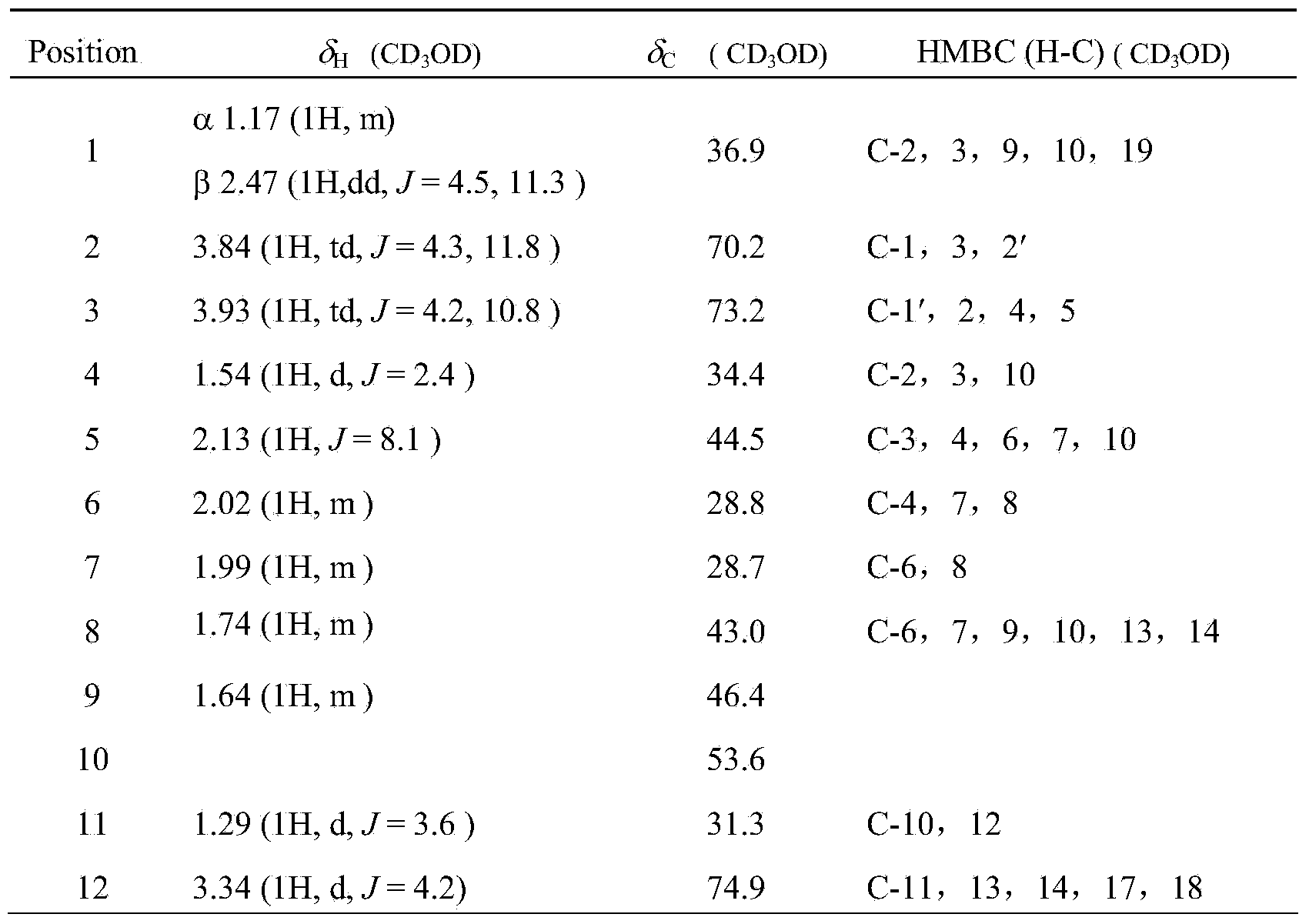
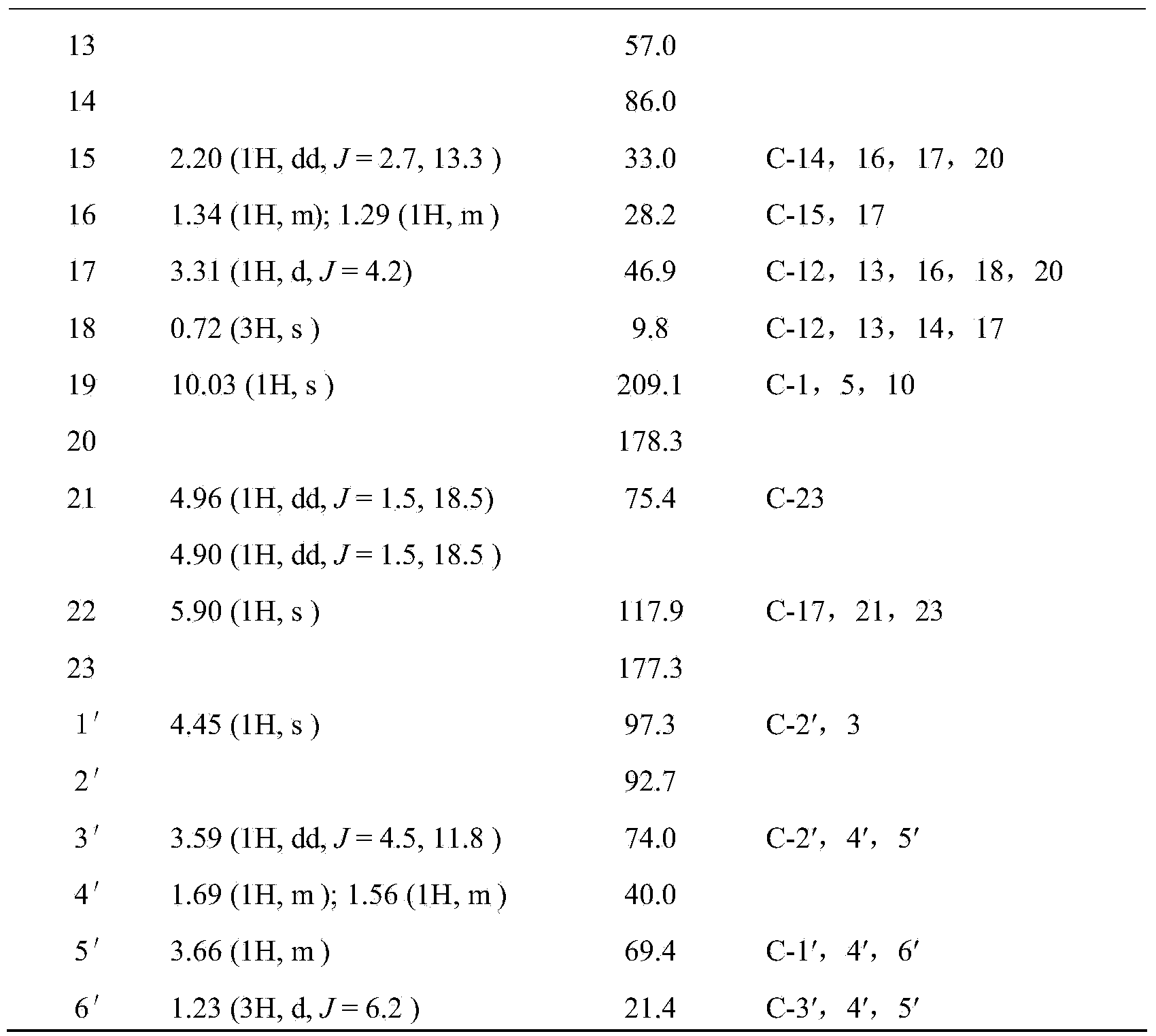
[0035] Structural identification of cardiac glycoside compound 12β-hydroxycalotropin:
[0036]The structure of the cardiac glycoside compound 12β-hydroxycalotropin in Example 1 was identified by spectroscopic techniques, including ultraviolet, infrared, nuclear magnetic resonance and high-resolution mass spectrometry. The detection of the cardiac glycoside compound 12β-hydroxycalotropin for the first time in the stem of the horn squash by using 2D-NMR technology 13 C-NMR, 1 The H-NMR data were assigned (see Table 1 below).
[0037] The following are the physical and chemical constants of the cardiac glycoside compound 12β-hydroxycalotropin:
[0038] 12β-hydroxycalotropin: C 29 h 40 o 10 , White amorphous powder (methanol), 10% sulfuric acid ethanol solution is brown. m.p.214-216℃; [α]D+6.4°(c=0.045, MeOH); HR-ESI-MS: m / z[M+Na] + 583.2318 (calcd. For C 29 h 40 o 10 Cl, 583.2310); IRλ max (cm -1 ):3433cm -1 ,2957cm -1 ,2923cm -1 ,2852cm -1 ,1712cm -1 ,1655cm -1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com