Polypeptide, preparation method and application thereof
A peptide chain and aspect technology, applied in the field of peptide preparation, can solve the problems of reversal and inability to cure the disease course, and achieve the effect of convenient operation and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The tissue extraction and preparation method (animal tissue extraction method) of the polypeptide of this embodiment includes the following steps:
[0036] 1) Extraction by acidified ethanol: take the skin of the northern leopard frog, mince it and digest it in acidified ethanol at 4°C for 12 hours, add 8 mL of acidified ethanol per gram of skin tissue (the acidified ethanol is made of ethanol with a concentration of 0.7mol / L Hydrochloric acid prepared according to the volume ratio of 3:1), centrifuged at 4°C and 3000g for 30min, and the supernatant was concentrated by rotation and freeze-dried to obtain a freeze-dried product;
[0037] 2) Separation by size exclusion chromatography: the lyophilized product obtained in step 1) was treated in acetic acid with a concentration of 2mol / L, and then loaded into a Sephadex G50 column (90×1.6cm) at a concentration of 2mol / L acetic acid balance, column flow rate 10mL / h, collect 60 components of the chromatographic peak, freeze-d...
Embodiment 2
[0041] The chemical synthesis preparation method (artificial synthesis method) of the polypeptide of this embodiment includes the following steps:
[0042] 1) Using Wang resin (0.6mmol / g) as the starting resin, Fmoc solid-phase synthesis method was used to couple each protected amino acid one by one. The condensing agent used in the coupling was 2-(1H-benzotrisazo L-1 -base)-1,1,3,3-tetramethyluronium tetrafluoroborate, synthesized to obtain a peptide chain resin with fully protected side chains;
[0043] 2) Cleave the peptide chain resin with fully protected side chains with a cleavage reagent, cleave the peptide chain from the resin and remove the side chain protection group to obtain a crude polypeptide; the cleavage reagent is trifluoroacetic acid, phenol, water, The mixture that triisopropylsilane is formulated according to the volume ratio is 88:5:5:2;
[0044] 3) Dissolve the crude peptide in a mixture of dimethyl sulfoxide and water at a volume ratio of 2:8 to prepare...
Embodiment 3
[0057] In this example, the polypeptide obtained in Example 1 was used for animal experiments.
[0058] Materials and Methods:
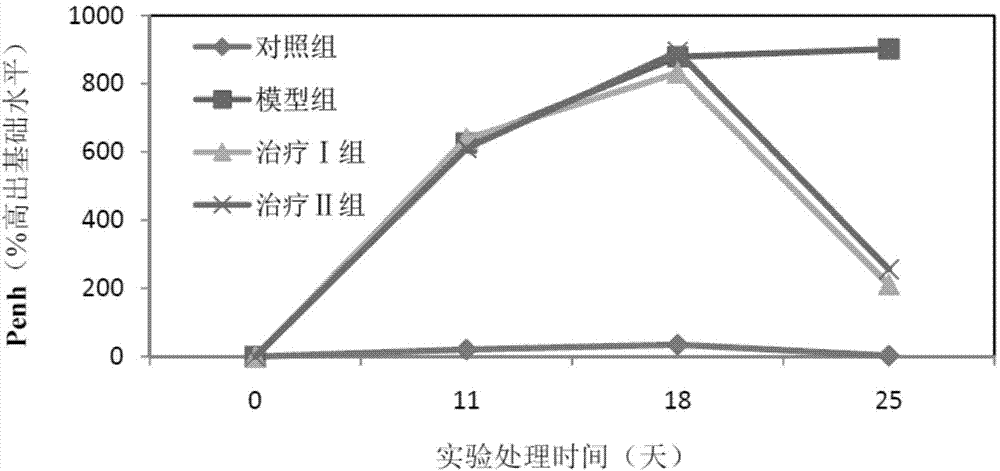
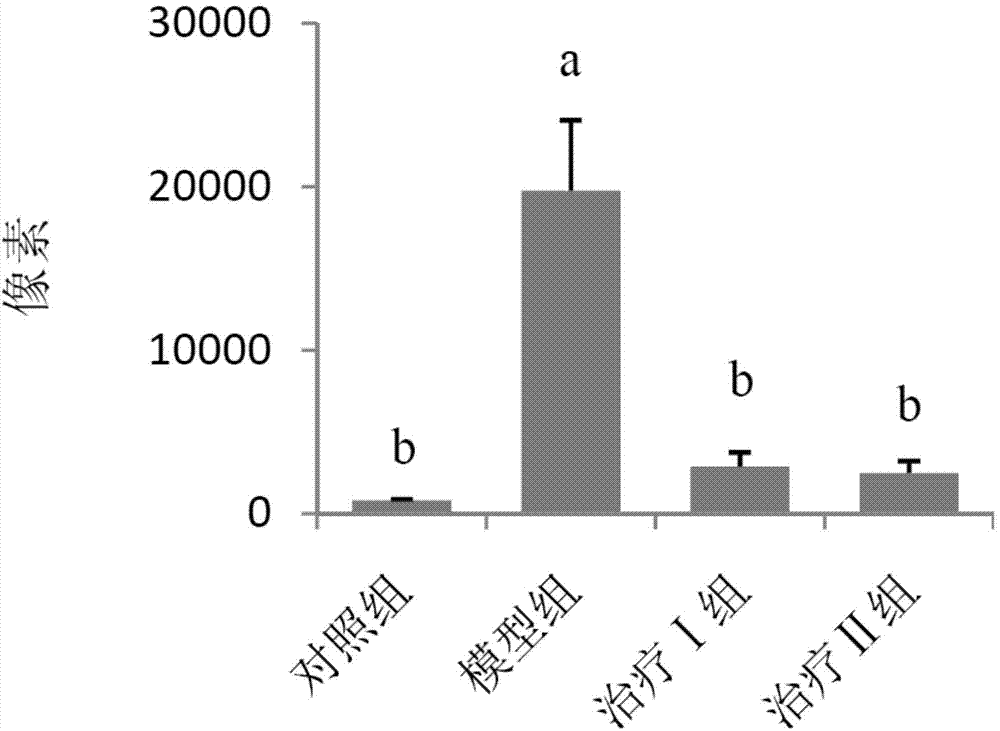
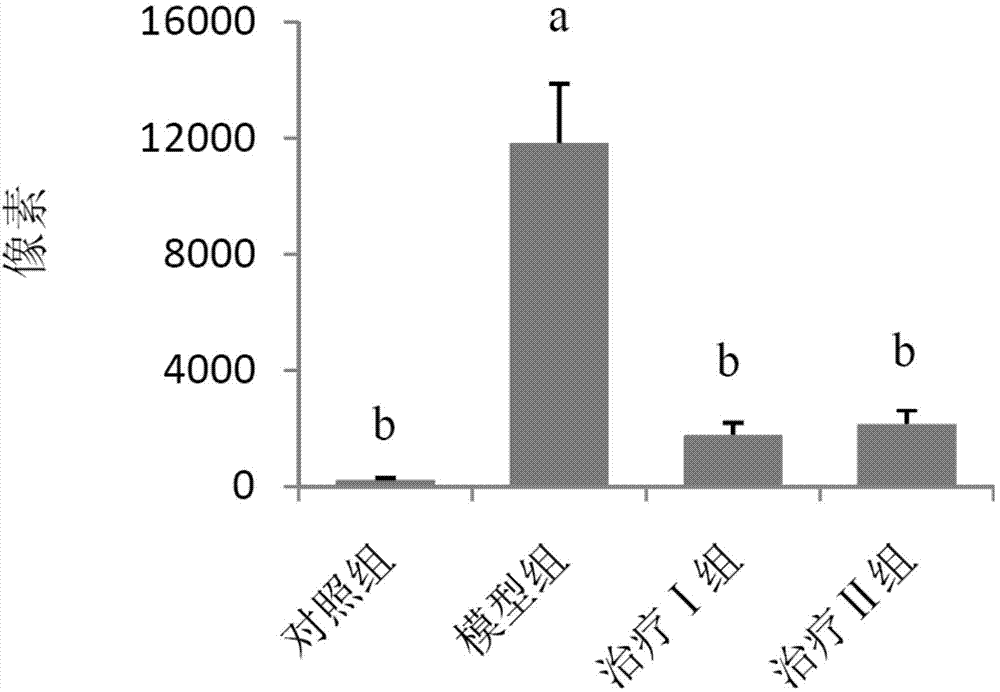
[0059] (1) Grouping of animals: Select 50 healthy female Wistar rats, weighing 120-140 g, and randomly divide them into 5 groups, namely control group, model group, treatment groups I, II and III, with 10 rats in each group, and raise them in single cages.
[0060] (2) Animal model of allergic rhinitis: on the first day, 1 mL of normal saline, 1.5 mg of ovalbumin, 2 mg of aluminum hydroxide sol and 1×10 10 Each inactivated Bacillus pertussis mixture was injected subcutaneously into rats at 0.2 mL each at 5 points. On the fifth day, the mixture was injected subcutaneously again except for pertussis. Rats were challenged from the 14th day: intranasally with 1% ovalbumin saline solution, 30 μL in each nostril, once a day, 10 times in total. Modeling was successful if the behavior (number of sneezing and nose scratching) and symptoms (nasal secretions...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Linear gradient | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com