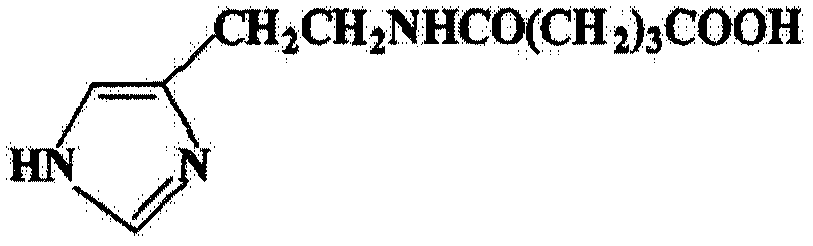
Agent for the treatment of hepatitis C virus
一种丙型肝炎、慢性丙型肝炎的技术,应用在医药领域,能够解决未提出戊二酸单酰基组胺病毒性肝炎、不知道戊二酸单酰基组胺组合等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] The aim of this study was to determine the antiviral activity of glutaryl histamine in an experimental model of hepatitis C virus infection in cell culture.
[0037] This study was performed in a cytopathic subtype 1b hepatitis C virus (HCV) strain. The strain was isolated from the serum of a patient with chronic hepatitis C and identified as a hepatitis C virus. The infectious HCV dose used was equal to 10.0 tissue cytopathic dose (TCID 50 ) / 20 μL.
[0038] Primary fibroblasts, which are highly sensitive to the cytopathic effects of HCV, were obtained after trypsinization of 9-day-old chicken embryos (FEC), and cultures of porcine embryonic kidney cells (SPEV) were obtained from Collection of Cell Cultures of the Institute of Virology RAMS. They are used as 1-day monolayers of cells grown in 24-well plastic plates. SPEV and FEC cell cultures were grown in Medium 199 with 10% fetal calf serum, supplemented with glutamine and antibiotics (100 U / ml).
[0039] The glu...
Embodiment 2
[0060] The aim of this study was to determine the clinical and laboratory effects of glutaryl histamine in patients with hepatitis C virus during combination antiviral therapy (AVT) with pegylated interferon and ribavirin. potency.
[0061] Patient inclusion criteria were as follows: 1) presence of symptoms of HCV infection in the patient - detection of anti-HVC antibodies by enzyme immunoassay (EIA); presence of HCV RNA of genotype 1b in serum (qualitative test); 2) general blood test WBC level was 3.9×10 9 / L to 3.0×10 9 / L, neutrophil level was 2.0×10 9 / L to 1.5×10 9 / L; 3) There is no hepatitis B surface antigen; 4) There is no antibody to human immunodeficiency virus; 5) There is no other clinically significant liver disease (alcoholic liver disease, administration of hepatotoxic drug preparations, autoimmune chronic hepatitis, hemochromatosis); and 6) the absence of cirrhosis.
[0062] Patients were administered a dose of 100 μg (or 1.5 μg / kg) of peg-interferon-α2b...
Embodiment 3
[0100] The aim of this study was to determine the effect of glutaryl histamine (dicarbamine) on the efficacy of antiviral therapy in patients with chronic HCV genotype 1b infection with initially reduced levels of leukocytes and neutrophils .
[0101] 86 patients with CHC (anti-HCV "+", HCV RNA "+", genotype 1b) aged between 20 and 52 years (mean age 32±1.2 years) were observed. Among them, 46 (53.5%) patients were male and 40 (46.5%) patients were female.
[0102] In order to achieve the aforementioned purpose, all patients were divided into two groups with comparable gender and age according to the treatment plan: Group 1 (n=44) administered peg-interferon-α2b+ribavirin; Group 2 (n=42) Receive peg-interferon-α2b + ribavirin + dicarbamine. Peg-interferon-α2b was administered subcutaneously at a dose of 100 μg (or 1.5 μg / kg) once a week, and ribavirin was administered daily at a dose of 1000 mg for 48 weeks. Dicarbamine was administered at a dose of 100 mg once a day for 9 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com