Poly(alpha-carbonyl diazo alkane) as well as preparation method and application thereof
A carbonyldiazoalkane, carbonyl technology, applied in the field of polymer synthesis, can solve the problems of inability to obtain polymers, limit the application of C1 polymer products, etc., and achieve the effect of strong fluorescence performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
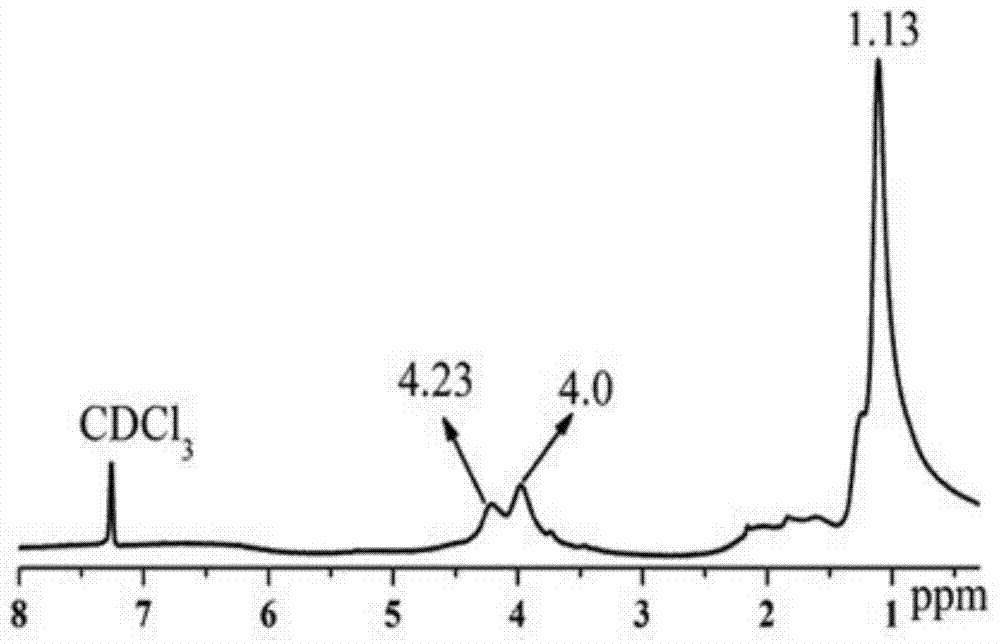
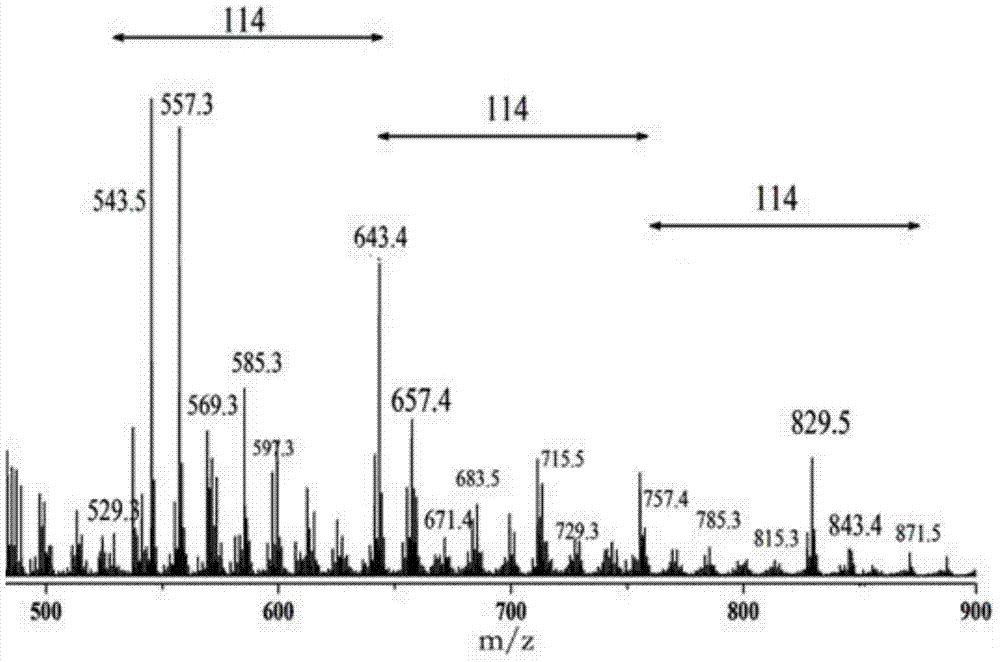
[0037]Under anhydrous and oxygen-free conditions, using chloroform as a solvent, add the catalyst poly(N-isopropylacrylamide-co-N-isopropenylimidazole) base-copper(Ⅱ) under the condition of constant stirring and feeding argon gas , adding ethyl diazoacetate dropwise at a mass ratio of catalyst to ethyl diazoacetate of 1:50, heating and reacting at 60°C for 24 hours, after the reaction, filter out the catalyst, and use diethyl ether as a precipitating agent to obtain A large amount of white flocculent precipitates were dried to obtain a poly(ethyl diazoacetate) polymer product, and the conversion rate of the polymer was calculated to be 95.40%.
Embodiment 2
[0039] Under anhydrous and oxygen-free conditions, using toluene as a solvent, adding poly(N-isopropylacrylamide-co-N-isopropenylimidazole)-copper(II) catalyst under the condition of constant stirring and feeding argon , add ethyl diazoacetate drop by drop according to the mass ratio of catalyst to ethyl diazoacetate at a ratio of 1:20, heat and react at 20°C for 16 hours, after the reaction is over, filter out the catalyst, and use n-hexane as a precipitant A white flocculent precipitate was obtained, and the polymer product was obtained after the precipitate was dried, and the conversion rate of the poly(ethyl diazoacetate) polymer was calculated to be 53.67%.
Embodiment 3
[0041] Under anhydrous and oxygen-free conditions, using tetrahydrofuran as a solvent, add poly(N-isopropylacrylamide-co-N-isopropenylimidazole)-copper(II) catalyst under the condition of constant stirring and flowing argon gas , add ethyl diazoacetate dropwise at a ratio of 1:70 by weight of the catalyst and ethyl diazoacetate, heat and react at 60°C for 18 hours, after the reaction, filter out the catalyst, and use petroleum ether as a precipitant A white flocculent precipitate was obtained, and the polymer product was obtained after the precipitate was dried, and the conversion rate of the poly(ethyl diazoacetate) polymer was calculated to be 71.74%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com