ADC (antibody-drug conjugate) cation exchange chromatographic purification method
An antibody-drug-conjugated, cation-exchange technology, applied in the field of protein separation and purification, to achieve the effects of reducing production costs, controlling risks, expanding the scope of application, and being easy to operate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] A cation-exchange chromatography purification method for antibody-conjugated drugs, using cation-exchange chromatography to purify antibody-conjugated drugs, controlling the small molecule / antibody coupling ratio (DAR) and polymers, and the purification method includes the following steps:
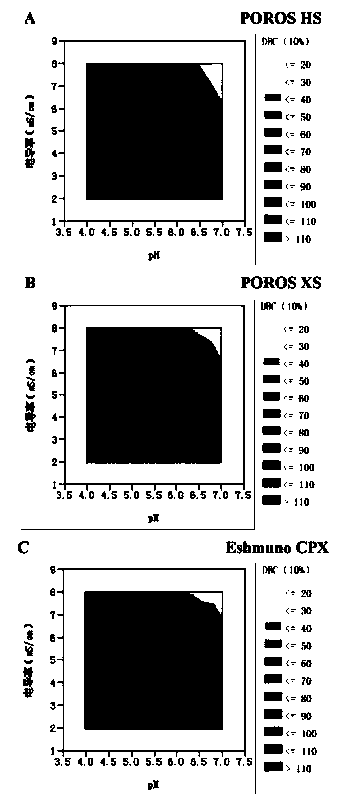
[0037] Adjust the ADC sample and equilibration buffer to pH 4-7, conductance 2-8 mS / cm, and select bind-elute mode or overload mode for purification.
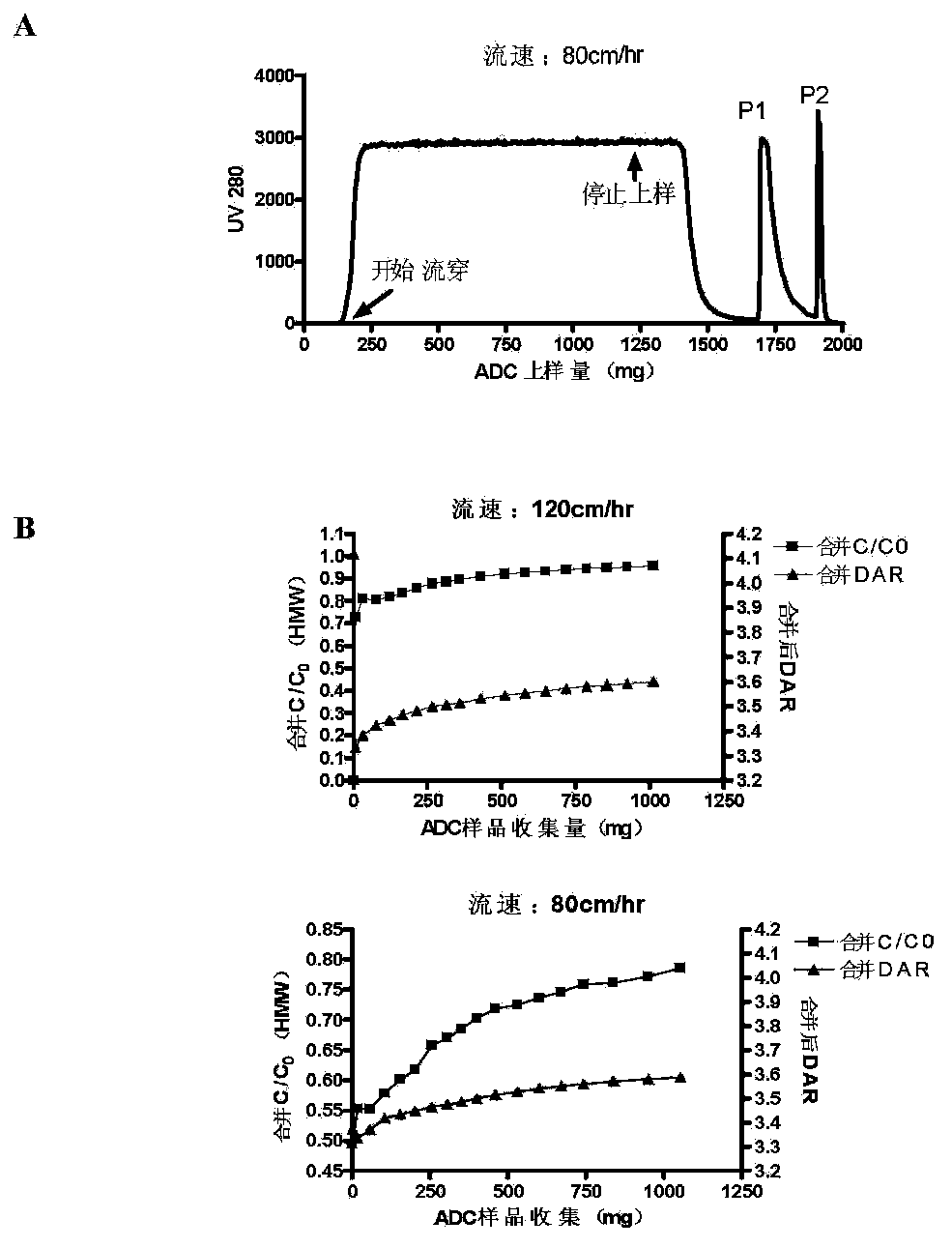
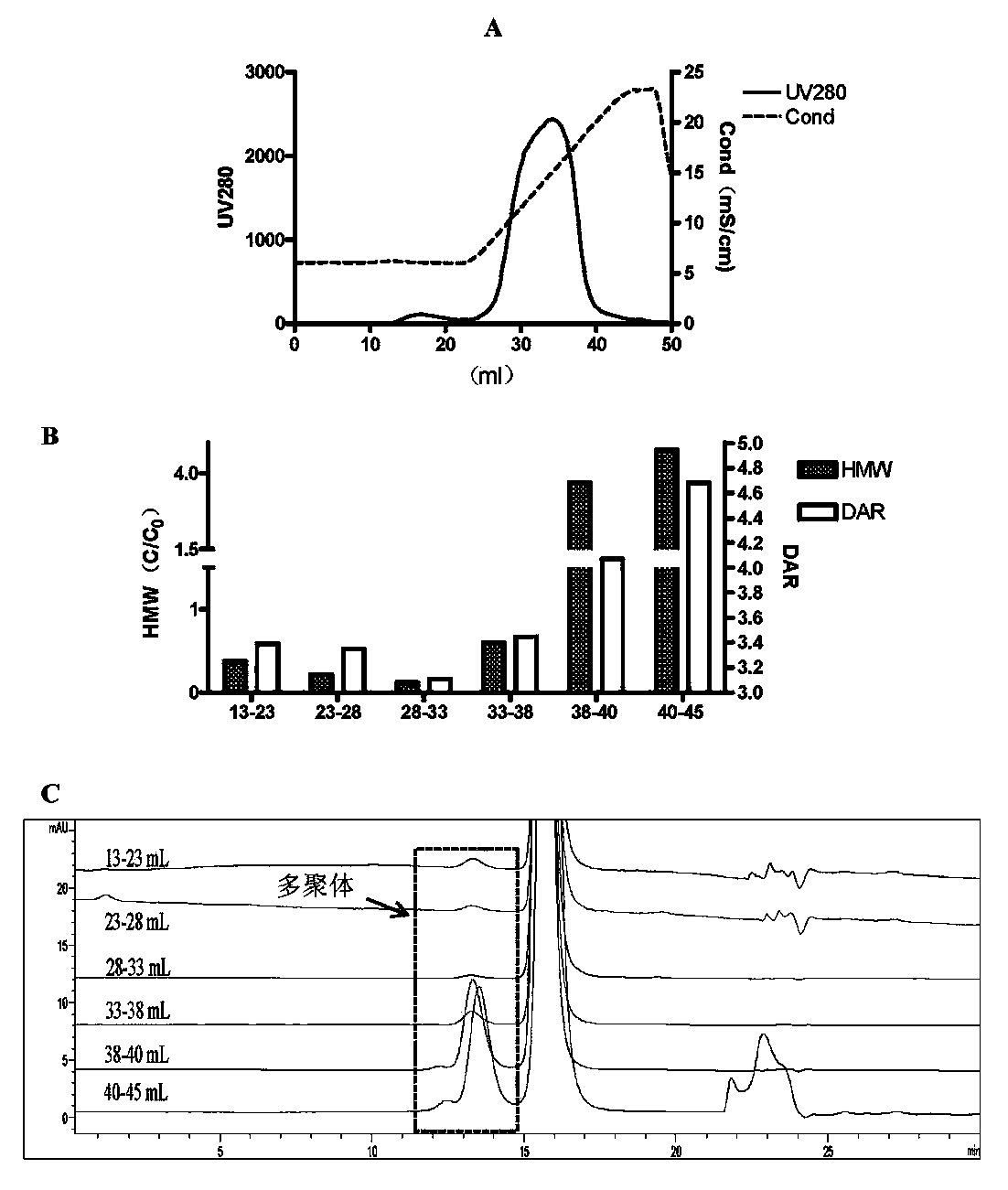
[0038] Further, the loading amount of the overload mode is not less than 300 mg / mL medium; the process of the overload mode includes: equilibrating the buffer to balance the chromatography medium, and then loading the antibody-conjugated drug, and waiting for the ADC sample to be adsorbed After saturation and start of flow-through, the target protein flow-through with DAR value and multimer content meeting the requirements is collected, and impurity multimers and proteins with high DAR value are adsorbed on the chromatographic column....
Embodiment 2
[0049] Example 2 Preparation of antibody-drug conjugate (ADC)
[0050]Preparation of lysine-coupled ADC samples: Before performing the modification reaction, replace the antibody (Ab) stock solution with the modification reaction buffer solution. Add bifunctional cross-linking reagent SMCC (linker) to modify lysine, and the reaction lasts for 2-3 hours. After the modification reaction, the SMCC not linked to the antibody was removed by G25 Sephadex chromatography or tangential flow filtration (UF / DF) system, and the modified intermediate product Ab-MCC was replaced with the coupling buffer ( pH 5-6). Then add the small molecule toxin DM1, couple with the linker on the antibody, and react for 3-15 hours to obtain a spare lysine-coupled ADC sample (Ab-DM1). The DAR value measured by UV spectrophotometry is 3.7-3.8 .
[0051] Preparation of cysteine-coupled ADC samples: Replace Ab stock solution into reaction buffer (pH 7-8). Add the reducing agent TCEP to react for 1-2 hou...
Embodiment 3
[0053] Embodiment 3 DAR value is measured
[0054] The obtained ADC sample was prepared in Example 2, and the coupling ratio of small molecule drug / antibody was determined by ultraviolet (UV) spectrophotometry. According to the different extinction coefficients of antibodies and drugs at different wavelengths, the drug-antibody coupling ratio (DAR) was derived from the binary linear equation. Specifically: dilute the antibody conjugate to 0.3-0.5 mg / mL, and scan the absorbance of the diluted ADC sample at a wavelength of 230-330 nm. The maximum absorption peak of the antibody is at 280 nm, the maximum absorption peak of the small molecule DM1 is at 252 nm, and the maximum absorption peak of the small molecule MMAE is at 248 nm. For the Ab-DM1 sample, the absorbance at 252 nm and 280 nm were respectively selected, and for the Ab-MMAE sample, the absorbance at 248 nm and 280 nm were respectively selected. According to the literature (Yu Chuanfei et al. Drug in an antibody dru...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Conductance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com