Chitosan semi-fluid slow-release gel and its application
A slow-release gel and chitosan technology, applied in prosthetics, liquid delivery, medical science, etc., can solve the problem of easy inactivation of water-soluble protein drugs and achieve good biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] A chitosan semi-fluid slow-release gel is made from the following components:
[0023] Component 1: a chitosan solution with a concentration of 0.5020g / ml prepared with physiological saline as a solvent, the molecular weight of chitosan is 5kDa, the degree of deacetylation is 95%, and it is obtained by dissolving at 4°C;
[0024] Component 2: a hyaluronic acid chitosan solution with a concentration of 0.2510 g / ml was prepared by using a mixture of saturated sodium bicarbonate aqueous solution and distilled water with a volume ratio of 1:1 as a solvent, and the hyaluronic acid chitosan solution was The molecular weight is 500kDa, the intrinsic viscosity is 3000g / ml, and it is obtained by dissolving at 4℃.
[0025] Before use, mix component 1 and component 2 in a 1:1 volume ratio at room temperature to form a semi-fluid gel, see figure 1 A, available for injection. The mixture was heated to 37 °C for 3 hours, and it was in a solidified state, see figure 1 B.
[0026] ...
Embodiment 2
[0030] A chitosan semi-fluid slow-release gel is made from the following components:
[0031] Component 1: a chitosan solution with a concentration of 0.5020g / ml prepared with distilled water as a solvent, the molecular weight of chitosan is 5kDa, the degree of deacetylation is 95%, and it is obtained by dissolving at 4°C;
[0032] Component 2: a hyaluronic acid chitosan solution with a concentration of 0.2510 g / ml was prepared by using a mixture of saturated sodium bicarbonate aqueous solution and distilled water with a volume ratio of 1:1 as a solvent, and the hyaluronic acid chitosan solution was The molecular weight is 500kDa, the intrinsic viscosity is 3000g / ml, and it is obtained by dissolving at 4℃.
[0033] Component 1 and Component 2 are mixed in a volume ratio of 1:1 at room temperature before use to form a semi-fluid gel, ready for injection.
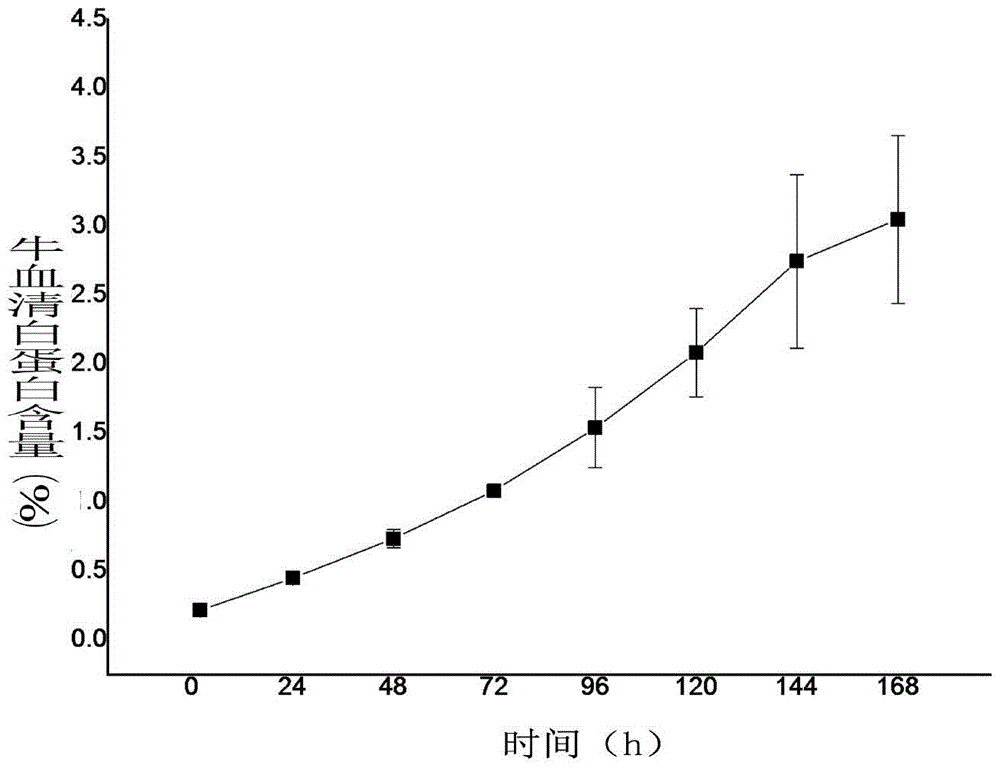
[0034] The method for fluorescent labeling of bovine serum albumin is to dissolve 20 mg of protein in 5 mL of carbonate bu...
Embodiment 3
[0038]A chitosan semi-fluid slow-release gel is made from the following components:
[0039] Component 1: a chitosan solution with a concentration of 0.5020g / ml prepared with physiological saline as a solvent, the molecular weight of chitosan is 3kDa, the degree of deacetylation is 65%, and it is obtained by dissolving at 4°C;
[0040] Component 2: The mixture of saturated sodium bicarbonate aqueous solution and distilled water with a volume ratio of 1:1 is used as a solvent to prepare a hyaluronic acid chitosan solution with a concentration of 0.2510g / ml. The molecular weight of hyaluronic acid chitosan It is 500kDa, and the intrinsic viscosity is 3000g / ml obtained by dissolving at 4°C.
[0041] Before use, mix component 1 and component 2 in a volume ratio of 1:1 at room temperature to form an injectable semi-fluid state.
[0042] Experiments have shown that lipid-soluble drugs with properties similar to paclitaxel: rapamycin, methotrexate, fluorouracil, mercaptopurine, hydr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com