Complexes of germanium with amino acids and carboxylic acids and method for preparing same
A technology of amino acids and coordination compounds, applied in germanium organic compounds, medical preparations containing active ingredients, pharmaceutical formulas, etc., can solve problems such as complex methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
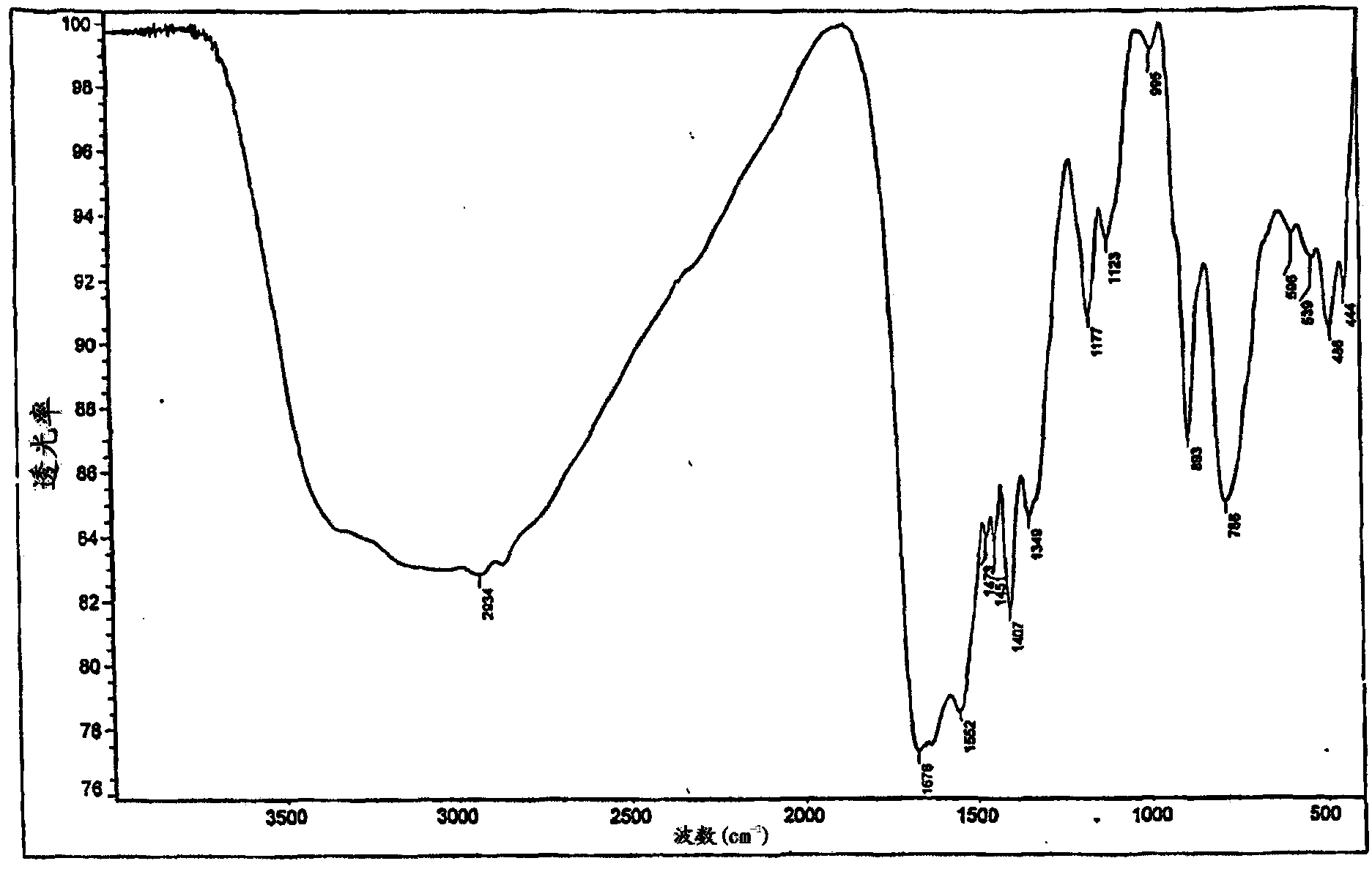
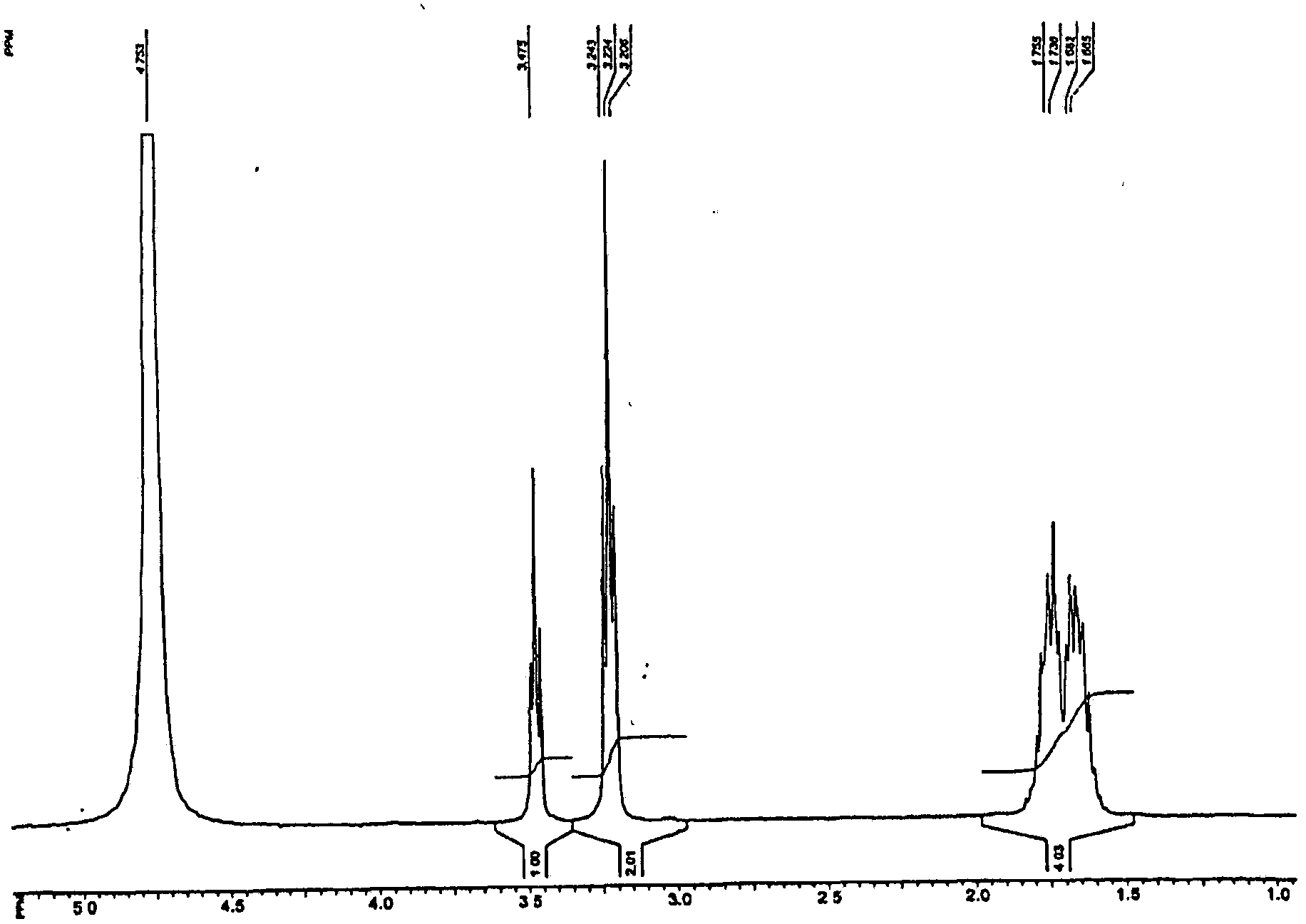
[0065] Add 3.12g (0.03mol) α-germanium dioxide GeO to a round bottom flask equipped with a stirrer and a thermometer 2, 5.22g (0.03mol) arginine HN=C (NH 2 )NH(CH 2 ) 3 CH(NH 2 ) COOH and 150 mL distilled water. The suspension was stirred under heating (at 85-95° C.) for 2 hours. The resulting clear solution was cooled, filtered and the water was removed on a rotary evaporator. The product was obtained as 8.4 g (94%) of a white amorphous powder. The IR and NMR spectra of the product compounds are shown in Figures 1a and 1b. Elemental analysis data are presented in Table 1. Elemental analysis and spectral data showed that the product was equivalent to compound (II).
Embodiment 2
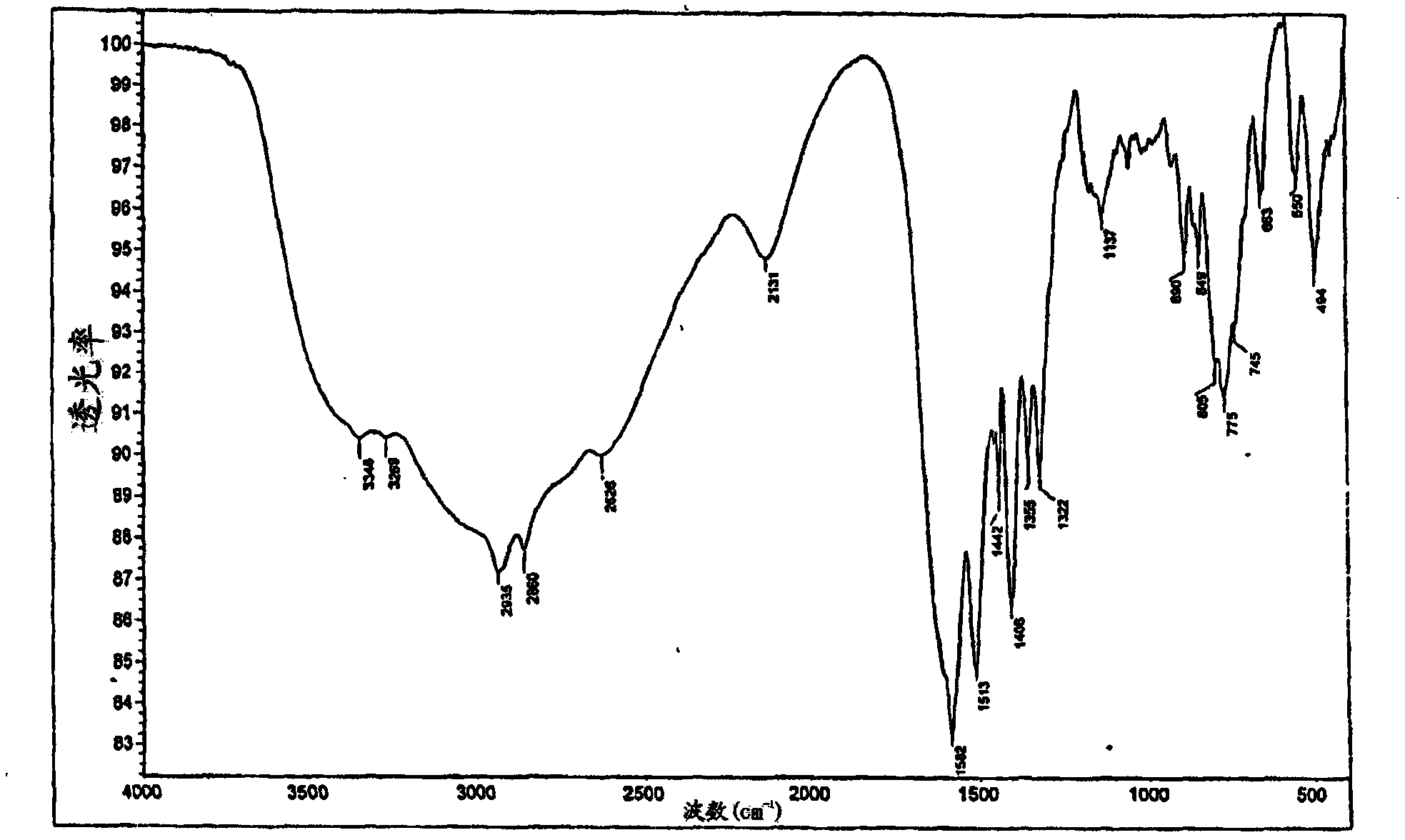
[0067] Add 3.12g (0.03mol) α-germanium dioxide GeO to a round bottom flask equipped with a stirrer and a thermometer 2 , 9.84g (0.06mol) lysine monohydrate H 2 N(CH 2 ) 4 CH(NH 2 )COOH·H 2 O and 200 mL of distilled water. The suspension was stirred with heating (at 85-95° C.) for 2 hours until a clear solution formed. The resulting clear solution was then cooled, filtered and the water removed using a rotary evaporator. The product was obtained as 11.4 g (96%) of a white amorphous powder. The IR and NMR spectra of the product compounds are shown in Figures 2a and 2b. Elemental analysis data are presented in Table 1. Elemental analysis and spectral data showed that the product was equivalent to compound (III).
Embodiment 3
[0069] Add 3.12g (0.03mol) α-germanium dioxide GeO to a round bottom flask equipped with a stirrer and a thermometer 2 , 10.71g (0.09mol) threonine CH 3 CH(OH)CH(NH 2 )COOH and 300mL distilled water. The suspension was stirred with heating (at 90-100° C.) for 2 hours until a clear solution formed. The resulting clear solution was then cooled, filtered and the water removed using a rotary evaporator. The product was obtained as 12.4 g (97%) of a white amorphous powder. The IR and NMR spectra of the product compounds are shown in Figures 3a and 3b. Elemental analysis data are presented in Table 1. Elemental analysis and spectroscopic data showed that the product was equivalent to compound (IV).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com