Efficient synthesis and separation method of phytosterol ester
A phytosterol ester and separation method technology, applied in the field of high-efficiency synthesis and separation of phytosterol ester, can solve the problems of toxic solvents and food safety hazards, and achieve the effects of low freezing point, high safety, and low pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
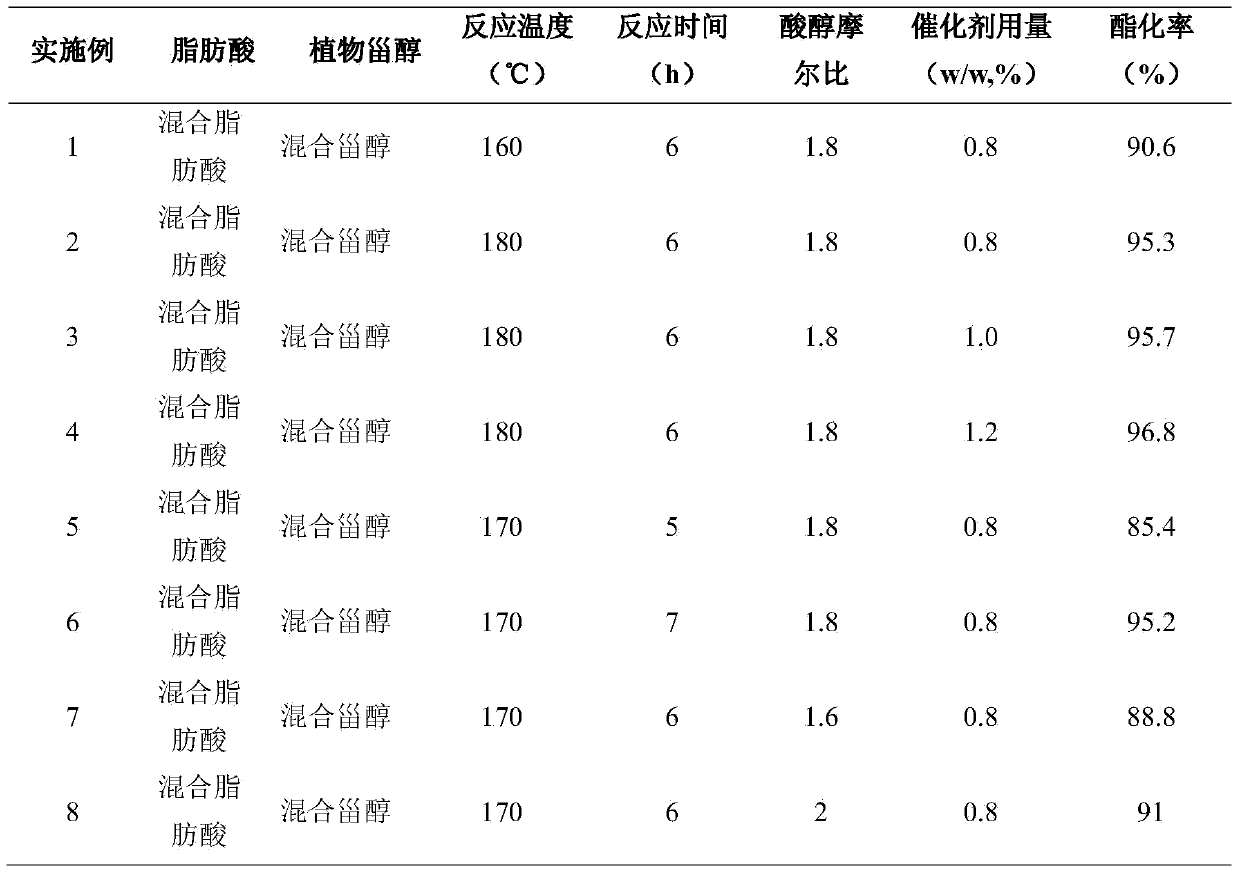
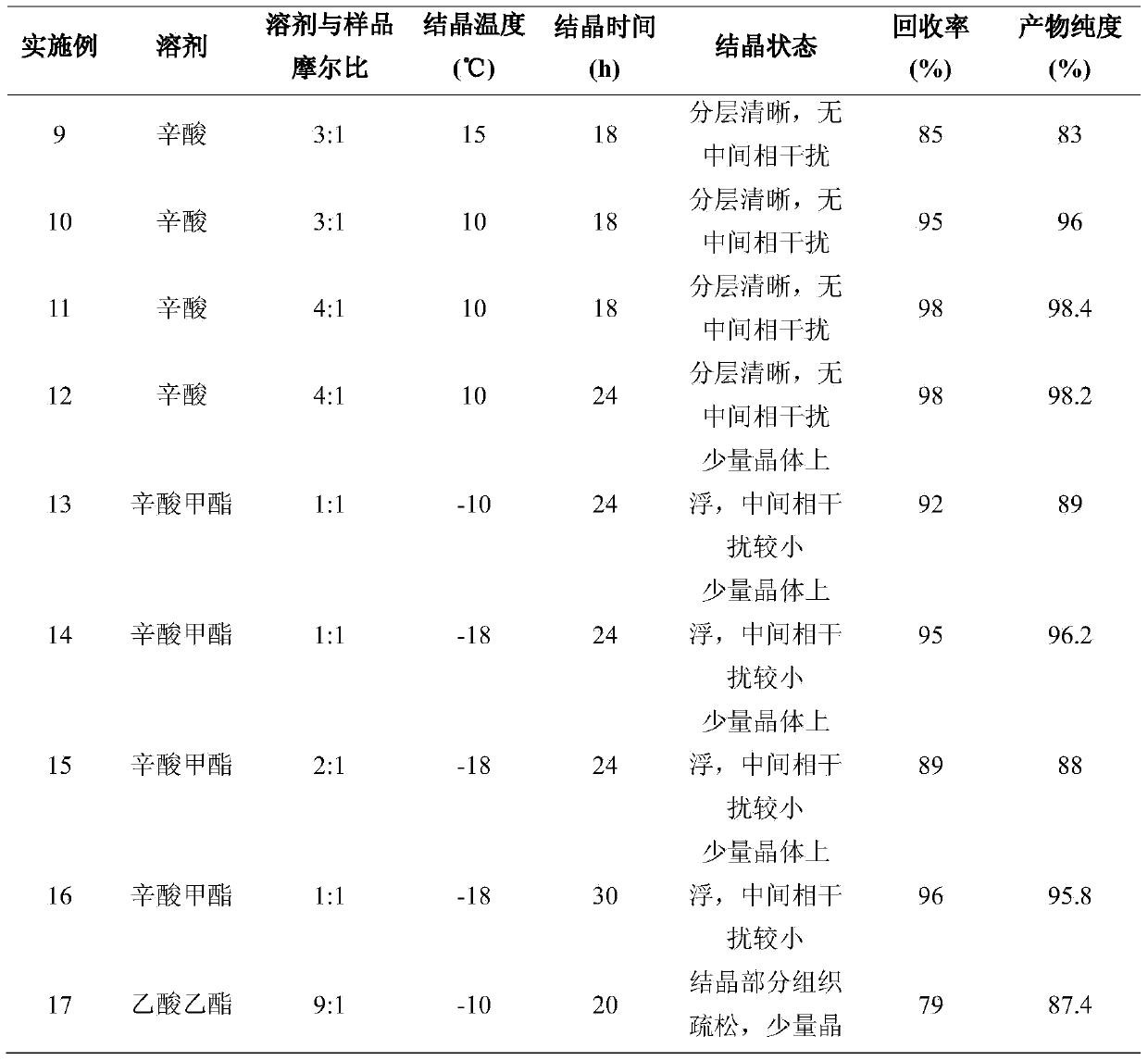
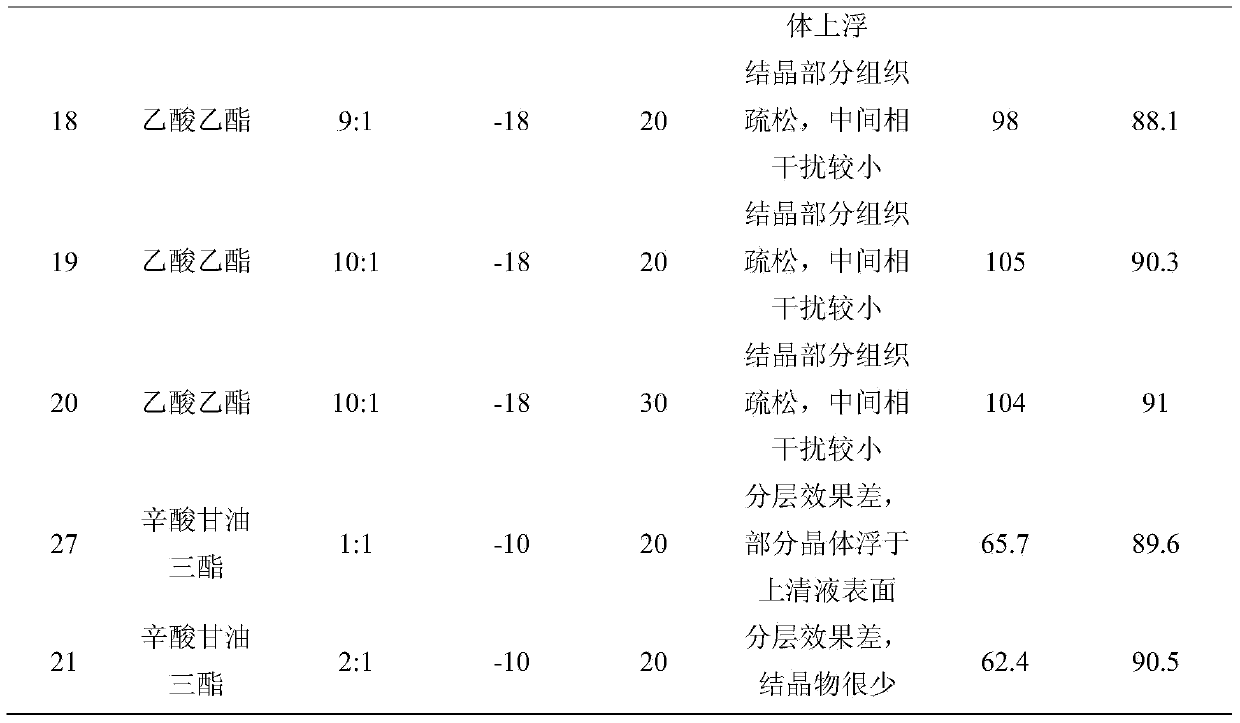
Embodiment 1~21
[0042] Step 1. Preparation of supported catalyst
[0043] Weigh 2g of NHAP (nano hydroxyapatite) carrier into a 100mL reactor, add 60mL of deionized water, at 120℃, pressure 1.2MPa, N 2 Under the protection conditions, the carrier steam expansion treatment for 2h. Then put it in a 110℃ vacuum oven to dry for 6 hours to remove moisture, and roast in a 600℃ muffle furnace for 4 hours to improve mechanical strength. After cooling to room temperature, vacuum for 30 minutes to remove the gas and moisture inside the porous material and facilitate the active assembly Points into the carrier channel.
[0044] Measure the saturated water absorption of the carrier first, and weigh the volume of 2g of NHAP that has been expanded and added to the water as V 1 , After immersing at room temperature for a period of time, use a straw to absorb excess water 2 , The saturated water absorption rate of every 2g carrier (V 1 -V 2 ) / 2, the amount of catalyst added is calculated based on the effective ac...
Embodiment 22~25
[0058] Using the same synthesis and separation method as in Example 4, the only difference is that after the reaction is completed, vacuum filtration is used to remove Cu(NO 3 ) 2 -NHAP, the separated Cu(NO 3 ) 2 -NHAP was cleaned by absolute ethanol and vacuum dried at 105°C and put into the next batch reaction. The above steps were repeated 4 times. After each reaction, the product esterification rate was used as an indicator to measure the change in catalyst activity, as shown in Table 3:
[0059] table 3
[0060]
[0061] Examples 22-25 refer to the changes in the esterification rate after one, two, three and four reactions under the optimal esterification reaction conditions, respectively. It can be seen that the repeated use of Cu(NO 3 ) 2 -After NHAP three times, the catalyst activity decreased significantly. The data showed that the reaction esterification rate fell below 80%, so the supported catalyst can be used repeatedly for at least three batch reactions.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com