Preparation method of terminal arylacetylenes
A technology of terminal alkynes and aryl groups, which is applied in the field of preparation of aryl terminal alkynes, can solve problems such as low yield and difficult separation, and achieve the effects of lowering reaction temperature, shortening reaction time, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Under nitrogen protection, 25 mL of triethylamine, 785 mg (5.0 mmol) of bromobenzene, 420 mg (5.0 mmol) of 2-methyl-3-butynyl-2-ol, and bis(triphenyl Phosphine) palladium dichloride (Pd(PPh 3 ) 2 Cl 2 )70mg (0.1mmol), triphenylphosphine (PPh 3 ) 105mg (0.4mmol), cuprous iodide (CuI) 38mg (0.2mmol), react at 65 ℃ until the thin-layer chromatography monitoring raw material bromobenzene spot disappears as the end point. The reaction solution was evaporated to dryness under reduced pressure, CH 2 Cl 2 Dissolved, washed successively with 5% dilute hydrochloric acid, saturated NaCl solution, anhydrous MgSO 4 Dry and filter. After the filtrate was evaporated to dryness under reduced pressure, the crude product was purified by column chromatography (eluent: petroleum ether: ethyl acetate = 10:1) to obtain 4-phenyl-2-methyl-3-butynyl-2- Alcoholic white solid 736mg (4.6mmol), yield 92%. 1 H NMR (CDCl 3 ) δ 7.40–7.43 (m, 2H), 7.28–7.30 (m, 3H), 2.07 (s, 1H), 1.62 (s, 6H);...
Embodiment 2
[0028] Under nitrogen protection, 25 mL of triethylamine, 1.415 g (5.0 mmol) of 4-bromoiodobenzene, 420 mg (5.0 mmol) of 2-methyl-3-butynyl-2-ol, and bis (Triphenylphosphine) palladium dichloride (Pd(PPh 3 ) 2 Cl 2 )70mg (0.1mmol), triphenylphosphine (PPh 3 ) 105mg (0.4mmol), cuprous iodide (CuI) 38mg (0.2mmol), reacted at 35 ℃ until the thin-layer chromatography monitoring raw material p-bromoiodobenzene spot disappears as the end point. The reaction solution was evaporated to dryness under reduced pressure, CH 2 Cl 2 Dissolved, washed successively with 5% dilute hydrochloric acid, saturated NaCl solution, anhydrous MgSO 4 Dry and filter. After the filtrate was evaporated to dryness under reduced pressure, the crude product was purified by column chromatography (eluent: petroleum ether: ethyl acetate = 10:1) to obtain 4-(4-bromophenyl)-2-methyl-3-butane Alkynyl-2-ol white solid 1.052g (4.4mmol), yield 90%. 1 H NMR (CDCl 3 ) δ 7.42 (d, J = 8.1 Hz, 2H), 7.26 (d, J ...
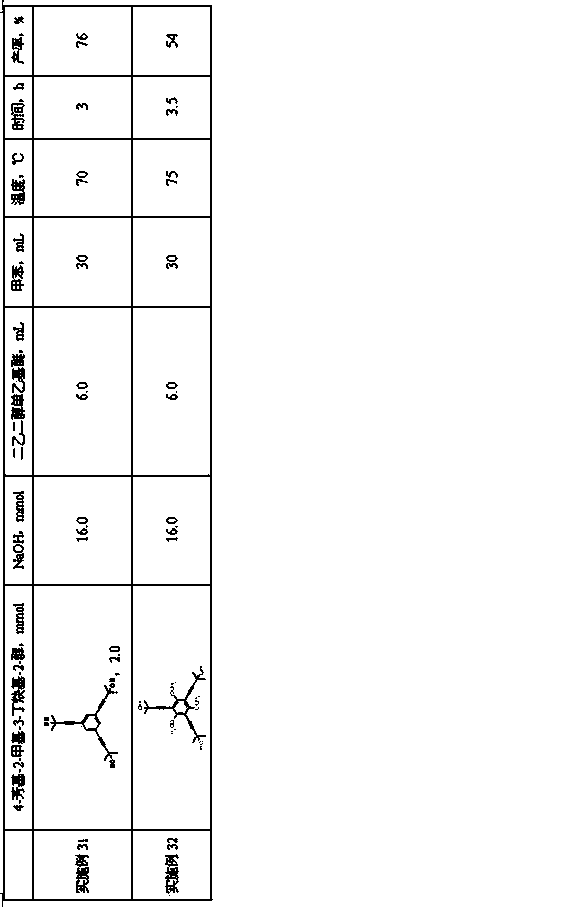
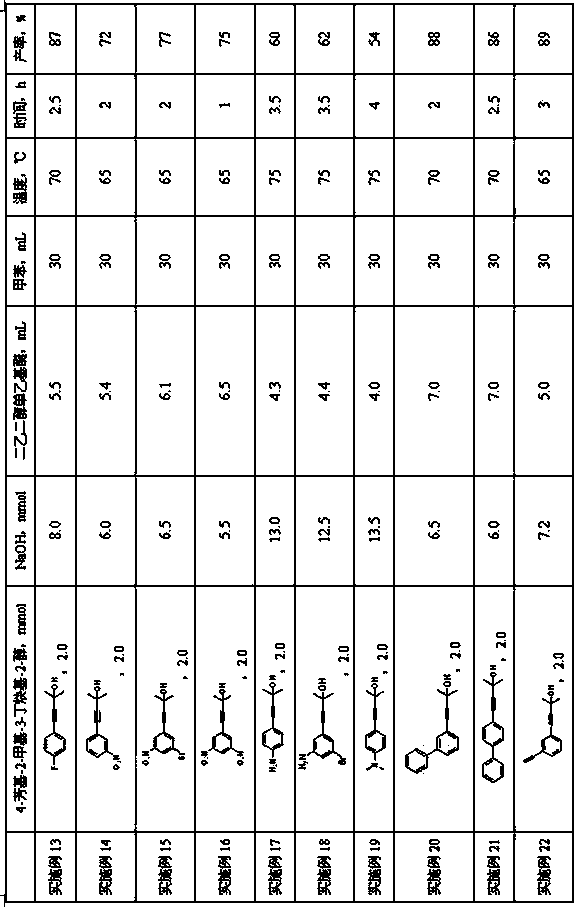
Embodiment 3~ Embodiment 32
[0033] The preparation method is the same as in Example 1, and the amount of each raw material and process parameters in the second step of deprotection group reaction are shown in Table 1.
[0034]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com