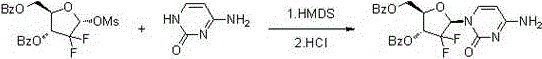A kind of preparation method of gemcitabine hydrochloride intermediate
A technology of white solid and cytosine, applied in the field of pharmacy, can solve problems such as safety hazards, equipment corrosion, DNA damage, etc., and achieve the effects of convenient operation, environmental friendliness, safe and green process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
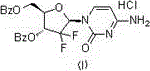
[0031] Put cytosine (22.2g, 0.2mol), hexamethyldisilazane (84mL, 0.4mol), and 0.10g ammonium sulfate in a 500mL three-neck flask, stir, heat and reflux until cytosine is completely dissolved and clarified, and then continue the insulation reaction After 0.5-1h, no ammonia gas is released. Then the temperature was lowered to 100° C. and the remaining hexamethyldisilazane was concentrated under reduced pressure to obtain a white solid of cytosine silyl ether protecting group. Add 100mL of isoamyl alcohol to a 500mL reaction flask, stir, heat to 70-80°C to dissolve the white solid, and transfer it to an insulated dropping funnel.
[0032] Add 2-deoxy-2,2-difluoro-D-erythro-pentafuranose-3,5-diphenylmethyl ester-1-methanesulfonate (45g, 0.1mol) and 0.5g to a 1L three-necked flask Phosphotungstic acid and 100mL isoamyl alcohol were stirred and heated to 128-132°C, and then the cytosine silyl ether protecting group was added dropwise. After 2.5 hours of dropwise addition, the tempe...
Embodiment 2
[0034] Put cytosine (22.2g, 0.2mol), hexamethyldisilazane (84mL, 0.4mol), and 0.10g ammonium sulfate in a 500mL three-neck flask, stir, heat and reflux until cytosine is completely dissolved and clarified, and then continue the insulation reaction After 0.5-1h, no ammonia gas is released. The temperature was lowered to 100° C. and the remaining hexamethyldisilazane was concentrated under reduced pressure to obtain a white solid of cytosine silyl ether protecting group. Add 100mL of isoamyl alcohol to a 500mL reaction flask, stir, heat to 70-80°C to dissolve the white solid, and transfer it to an insulated dropping funnel.
[0035] Add 2-deoxy-2,2-difluoro-D-erythro-pentafuranose-3,5-benzhydryl-1-methanesulfonate (45g, 0.1mol) and 0.8g to a 1L three-necked flask Phosphomolybdic acid and 100mL isoamyl alcohol were stirred and heated to 128-132°C, then the cytosine silyl ether protecting group was added dropwise, after 3 hours of dropping, the temperature was raised to reflux fo...
Embodiment 3
[0037] Put cytosine (22.2g, 0.2mol), hexamethyldisilazane (84mL, 0.4mol), and 0.10g ammonium sulfate in a 500mL three-neck flask, stir, heat and reflux until cytosine is completely dissolved and clarified, and then continue the insulation reaction After 0.5-1h, no ammonia gas is released. Then the temperature was lowered to 100° C. and the remaining hexamethyldisilazane was concentrated under reduced pressure to obtain a white solid of cytosine silyl ether protecting group. Add 100mL of n-pentanol to a 500mL reaction flask and stir, heat to 70-80°C to dissolve the white solid and transfer it to an insulated dropping funnel.
[0038] Add 2-deoxy-2,2-difluoro-D-erythro-pentafuranose-3,5-diphenylmethyl ester-1-methanesulfonate (45g, 0.1mol) and 1.0g to a 1L three-necked flask Phosphomolybdic acid and 100mL of n-amyl alcohol were stirred and heated to 128-137°C, then the cytosine silyl ether protecting group was added dropwise, after 2 hours of dropping, the temperature was raise...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com