Method for synthesizing sodium aluminum hydride by utilizing Grignard reagent method
A technology of Grignard reagent and sodium aluminum hydride, which can be applied to various metal hydrides and other directions, and can solve the problems of slow reaction rate, harsh reaction conditions of solid-phase method, and large energy consumption.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
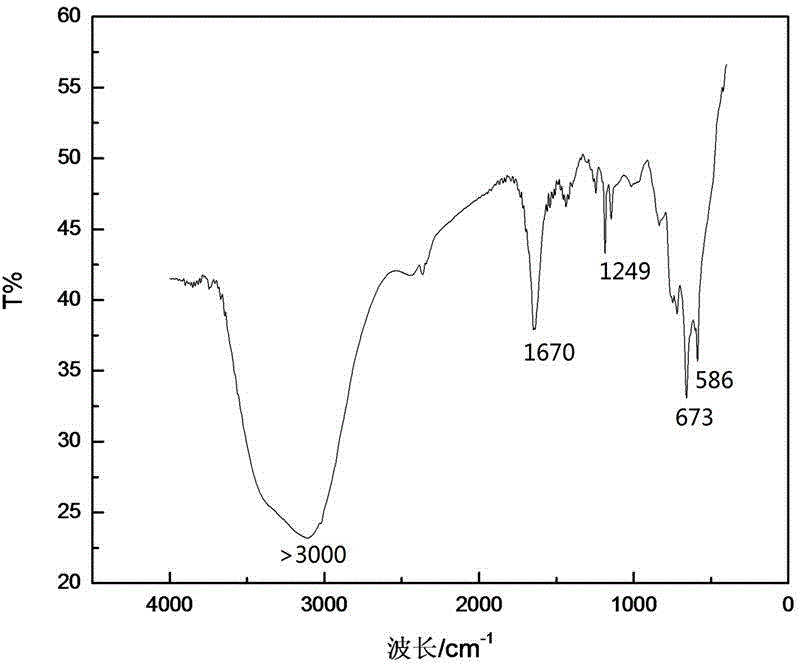
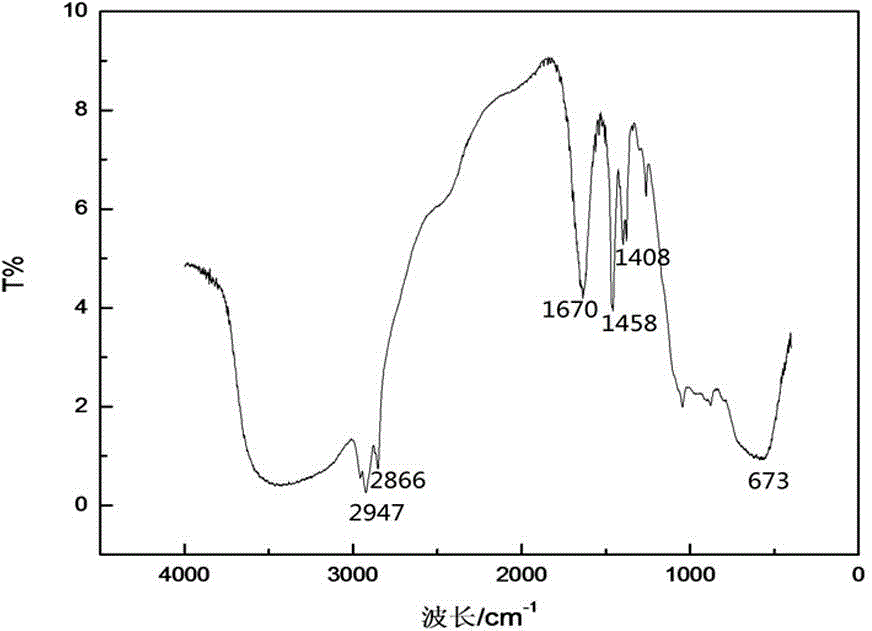
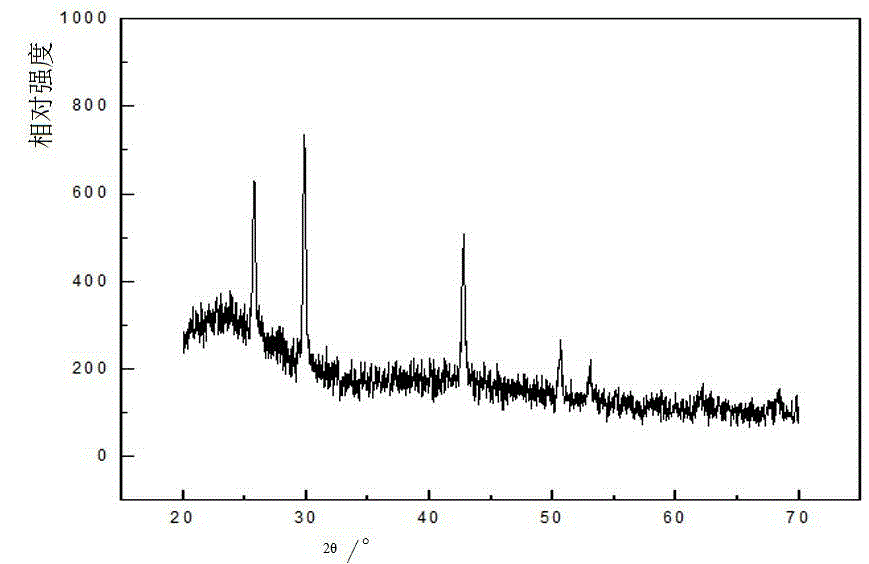
Image
Examples
Embodiment 1
[0037] Grignard reagent preparation method, the steps are:
[0038] (1) Weigh 1.00g of aluminum powder into the three-necked bottle, and protect it with hydrogen;
[0039] (2) Add 10ml of ethyl bromide to the bottle, set the temperature at 39°C, and react for 15 minutes;
[0040] (3) Observe the experimental phenomenon, if the reaction is slow or almost no reaction, add a small amount of iodine and anhydrous aluminum chloride to initiate the reaction;
[0041] (4) After the reaction occurs, condense the ethyl bromide to reflux, and set the temperature of the cooling circulating fluid below 0°C. At this time, white smoke in the system escapes through the exhaust device, and it is acidic when tested with pH test paper;
[0042] (5) After continuing to react for 1 hour, add a little sodium slice just cut into it, keep reflux, and react for another 6 hours;
[0043] (6) At this time, the system is a pasty black liquid, take out a little, smoke in the air, and react violently wit...
Embodiment 2
[0046] Utilize Grignard reagent to prepare sodium aluminum hydride, the steps are:
[0047] (1) Weigh 1.00g of aluminum powder into a three-necked bottle, and after protecting it with hydrogen, add iodine and anhydrous aluminum chloride;
[0048] (2) Then add 5ml bromoethane, set the temperature to 39°C for reaction;
[0049](3) After the reaction occurs, the ethyl bromide is condensed and refluxed, and the temperature of the cooling circulating fluid is set below 0°C, at this time, white smoke in the system escapes through the tail gas device;
[0050] (4) After continuing to react for 1 hour, add 3g of sodium hydride, keep reflux, and raise the temperature to 80°C;
[0051] (5) After continuing the reaction for 1 hour, add 10ml of toluene as a dispersant, and raise the temperature to 110°C, and continue the reaction for 5 hours;
[0052] (6) Adjust the temperature to 65°C, add 40ml tetrahydrofuran, and stir for 1 hour;
[0053] (7) After standing and stratifying, take the...
Embodiment 3
[0058] Utilize Grignard reagent to prepare sodium aluminum hydride, the steps are:
[0059] (1) Weigh 0.85g of aluminum powder into a three-necked bottle, and then add iodine and anhydrous aluminum chloride after hydrogen protection;
[0060] (2) Then add 5ml bromoethane, set the temperature to 39°C for reaction;
[0061] (3) After the reaction occurs, the ethyl bromide is condensed and refluxed, and the temperature of the cooling circulating fluid is set below 0°C, at this time, white smoke in the system escapes through the tail gas device;
[0062] (4) After continuing to react for 1 hour, add 3.5g of sodium hydride, maintain reflux, and raise the temperature to 80°C;
[0063] (5) After continuing the reaction for 1 hour, add 10ml of toluene as a dispersant, and raise the temperature to 110°C, and continue the reaction for 5 hours;
[0064] (6) Adjust the temperature to 65°C, add 40ml tetrahydrofuran, and stir for 1 hour;
[0065] (7) After standing and stratifying, take ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorbance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com