Polypeptide organic compound for interference in function of NMDA receptor and application of polypeptide organic compound
A technology of organic compounds and functions, applied in the field of chemistry, can solve the problems that the possibility of functional compensation cannot be ruled out, and it is difficult to correctly reflect functions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1 Obtaining the polypeptide of the present invention
[0022] Firstly, the ATD domain of GluN2A and the substrate binding domain of Bip were simulated using SWISS-MODEL workshop software. The ATD domain of GluN2A was simulated according to the structure of the homology domain of GluN2B (PDB database ID: 3jpw), and the substrate binding domain of Bip was simulated according to the homology domain of HSP70 (PDB database ID: 4JNF). Further, the interaction between these two domains was simulated, and the amino acids critical to Bip on the ATD domain of GluN2A were identified as points. According to the distribution of these critical points, three polypeptides were designed:
[0023] Polypeptide a is the 137th-147th amino acid, and the sequence is:
[0024] LEU-LYS-ILE-MET-GLN-ASP-TYR-ASP-TRP-HIS-VAL
[0025] Polypeptide b is amino acid 222-232, and its sequence is:
[0026] ARG-SER-LEU-GLY-LEU-THR-GLY-TYR-ASP-PHE-PHE
[0027] Polypeptide c is the 244th-256th ...
Embodiment 2
[0038] Example 2 Peptide interferes with the interaction between endogenous Bip and GluN2A in cultured neurons
[0039] The primary cultured rat cortical neurons were cultured in vitro for 14 days, and 20 μm polypeptide was added to the culture medium, and the culture was continued for 1 hour. Rinse the cells three times with pre-cooled HBSS, add pre-cooled lysate (50 mM Tris-HCl, 0.1 mM PMSF, 1 mM aprotinin, pH7.4) to lyse, and sonicate 8 times at 50% intensity. Add solution A (10% Deoxycholate, pH7.4) to the lysate by 1 / 10 volume, and incubate at 36 degrees for 30 minutes. Then add solution B (500 mM Tris.HCl, 1% Triton X-100, pH9.0) according to 1 / 10 of the volume of the lysate, blow and mix well, and then dialyze at 4 degrees overnight (dialysis solution composition: 50 mM Tris.HCl, 0.1% Triton X-100, pH7.4). During dialysis, the dialysate was changed 2-3 times. After dialysis, the sample solution was collected at 37000 x g and centrifuged at 4°C for 30 minutes. Ke...
Embodiment 3
[0040] Example 3 Peptide interferes with the interaction between endogenous Bip and GluN2A in the hippocampus
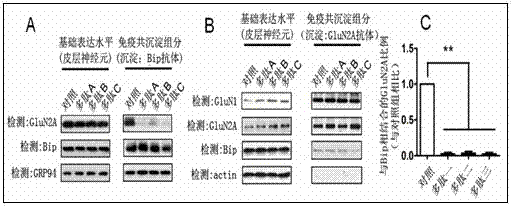
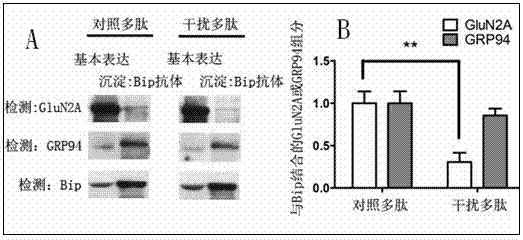
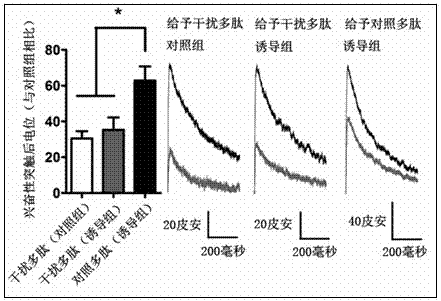
[0041] The mice were intraperitoneally injected with Polypeptide B at a dosage of 10 per kg . One hour after the injection, the hippocampal region of the mouse brain was taken, and 1 ml of pre-cooled lysate was added for every 0.5 g, and the brain tissue was lysed with a homogenizer. Centrifuge at 700 x g for 10 minutes, take the supernatant, add solution A (10% Deoxycholate, pH7.4) at 1 / 10 volume, and then detect the interaction between Bip and GluN2A according to the method of co-immunoprecipitation of cells. It was detected that Bip and GluN2A in the hippocampus of the mouse brain were effectively separated, and it was found that polypeptide B could effectively interfere with the interaction between GluN2A and Bip. The control polypeptide had no such effect. See results figure 2 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com