Traditional Chinese medicine Liandan anti-inflammatory tablet as well as preparation method and quality control method thereof
A technology of lotus bile anti-inflammatory tablets and lotus bile is applied in the directions of biochemical equipment and methods, microorganism-based methods, digestive systems, etc., and can solve the problems of unspecified tablet weight and unspecified addition amount.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0018] In order to make the objects and advantages of the present invention clearer, the present invention will be further described in detail below in conjunction with the examples. It should be understood that the specific embodiments described here are only used to explain the present invention, not to limit the present invention.
[0019] 1. Product overview
[0020] Drug name: Liandan Xiaoyan Tablets
[0021] Dosage form: tablet
[0022] Specifications: Each plain tablet weighs 0.23g
[0023] Properties: This product is a sugar-coated tablet, brownish green after removing the sugar coating; taste bitter
[0024] Storage: sealed
[0025] Validity period: 18 months
[0026] Approval number: Z20063563.
[0027] 2. Prescription and basis and preparation method:
[0028] 1. Prescription
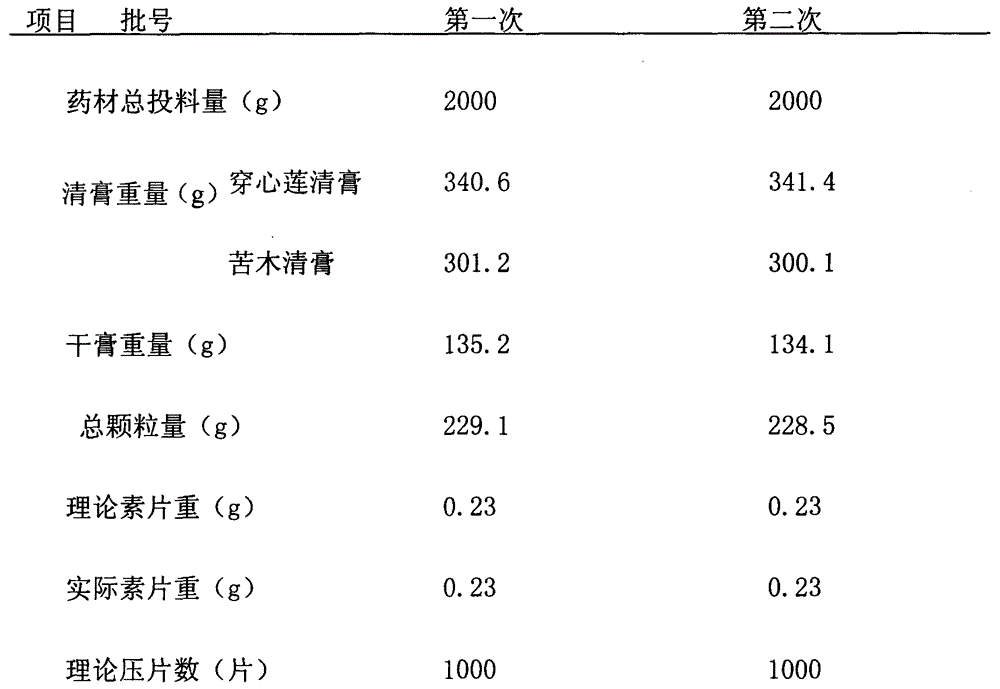
[0029] Andrographis paniculata 1000g, bitter wood 1000g, make 1000 pieces
[0030] 2. Preparation method
[0031] The above two flavors were crushed into coarse powder respectively,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com