ELISA (Enzyme-Linked Immunosorbent Assay) kit for detecting human anti-rabies virus antibody
A technology of rabies virus and kit, which is applied in the field of detection of antibodies in human blood samples, which can solve the problems of serious non-specific binding in ELISA detection, increased background of negative detection, low accuracy and sensitivity, and achieve small errors , reduce non-specific binding and improve accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1, the preparation of the ELISA kit that detects human anti-rabies virus antibody
[0021] (1) Preparation of reagents
[0022] Coating buffer: 0.1M carbonate buffer, pH9.6, weighed Na 2 CO 3 3.18g, NaHCO 3 5.88g, dilute to 1000ml with ultrapure water.
[0023] PBS buffer (pH7.4): 0.02M PBS buffer, pH7.4, weighed Na 2 HPO 4 12H 2 O 5.16g, NaH 2 PO 4 2H 2 O 0.87g, NaCl 7.6g, dilute to 1000ml with ultrapure water.
[0024] Plate washing solution (PBST): PBS buffer (pH7.4) containing 0.05% Tween-20.
[0025] Blocking solution: PBS buffer (pH7.4) containing 1% BSA.
[0026] Eluent: 300 mmol / L acetic acid solution.
[0027] Neutralizing solution: 1mol / L Tris-HCl buffer (pH8.0) containing 2% BSA.
[0028] Stop solution: 2 mol / L H 2 SO 4 solution.
[0029] Substrate: TMB A, B chromogenic solution.
[0030] (2) Preparation method of NM57 mouse monoclonal antibody S-230 and S-271
[0031] The amino acid sequence of the S-230 light chain variable ...
Embodiment 2
[0038] Embodiment 2, use kit to detect the plasma concentration of human anti-rabies virus NM57 monoclonal antibody
[0039] (1) Capture-elute NM57 monoclonal antibody in serum (plasma) specimen
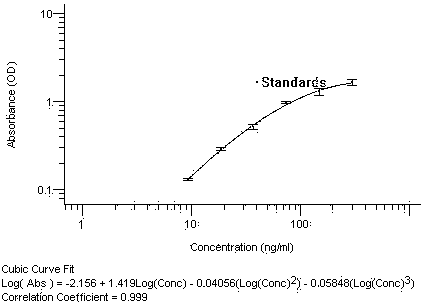
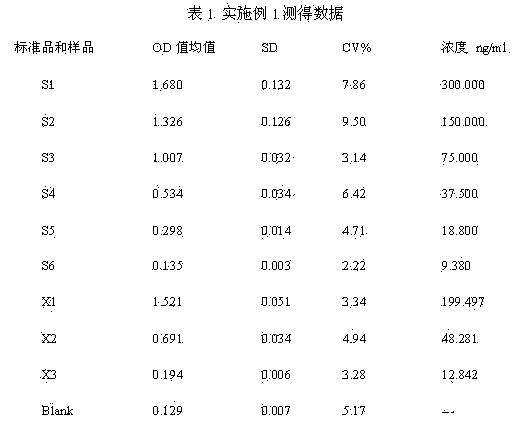
[0040] ① Capture target antibody: Add 40 μl of blocking solution to each well of the antibody capture plate, then add reference standards S1-S6, high, medium and low concentration quality controls, and serum samples to be tested, and incubate at 37°C for 2 hours with shaking. The preparation method of the reference standard S1~S6 is to dilute NM57 to 300 ng / ml with normal human serum to obtain S1, then double-dilution with normal human serum to obtain S2 (150 ng / ml), and sequentially double-dilute to S3 (75ng / ml). ml), S4 (37.5ng / ml), S5 (18.8ng / ml), S6 (9.38ng / ml); the preparation method of high, medium and low concentration quality control products is to dilute NM57 to 225 ng / ml with normal human serum Concentration quality control product X1, then diluted 4 times with normal huma...
Embodiment 3
[0050] Embodiment 3, test kit detection methodology verification result
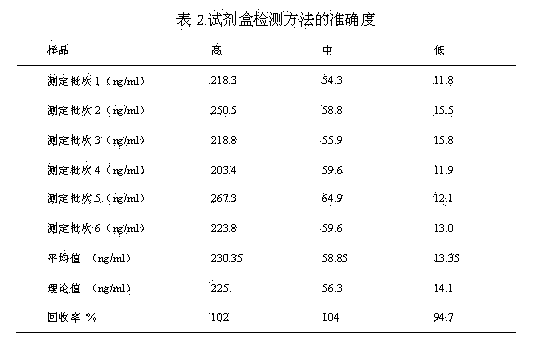
[0051] (1) Accuracy:
[0052] Six batches of high, medium and low samples (X1, X2, X3) were tested, and the recovery rate was calculated.
[0053]
[0054] The test results showed that the recoveries of high, medium and low concentration standards were 90%-110%.
[0055] (2) Accuracy (repeatability)
[0056] In one batch of determination, carry out the determination of 6 repeated samples for the high, medium and low samples (X1, X2, X3), and calculate the CV% value.
[0057]
[0058] The test results showed that the intra-plate repeatability (intra-assay precision) of high, medium and low concentration standards was 5.2%-6.4%.
[0059] (3) Accuracy (intermediate precision)
[0060] Three batches of high, medium and low samples (X1, X2, X3) were measured, and the CV% value was calculated.
[0061]
[0062] The test results showed that the inter-plate repeatability (inter-assay precision) of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com