Hydrolysable impurity compound of valsartan and preparation method, detection method and use thereof
A detection method and compound technology, applied in the field of medicine, can solve the problems of undisclosed impurity preparation methods and structural confirmation data, undisclosed application of hydrolyzed impurities, etc., to achieve good control and improve quality standards
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1 Preparation of valsartan hydrolysis impurity compound
[0040] (1) Get 30g of valsartan, dissolve it in 200ml of acetonitrile, add 200ml of 6mol / L hydrochloric acid, heat and reflux for 4 hours, stir and drop to room temperature, then add 50% NaOH aqueous solution to adjust pH to neutral, separate phases, Add NaCl to the obtained aqueous phase to become a saturated solution, filter, extract the obtained saturated aqueous solution with n-butanol, take the organic phase, and evaporate to dryness to obtain a white solid;
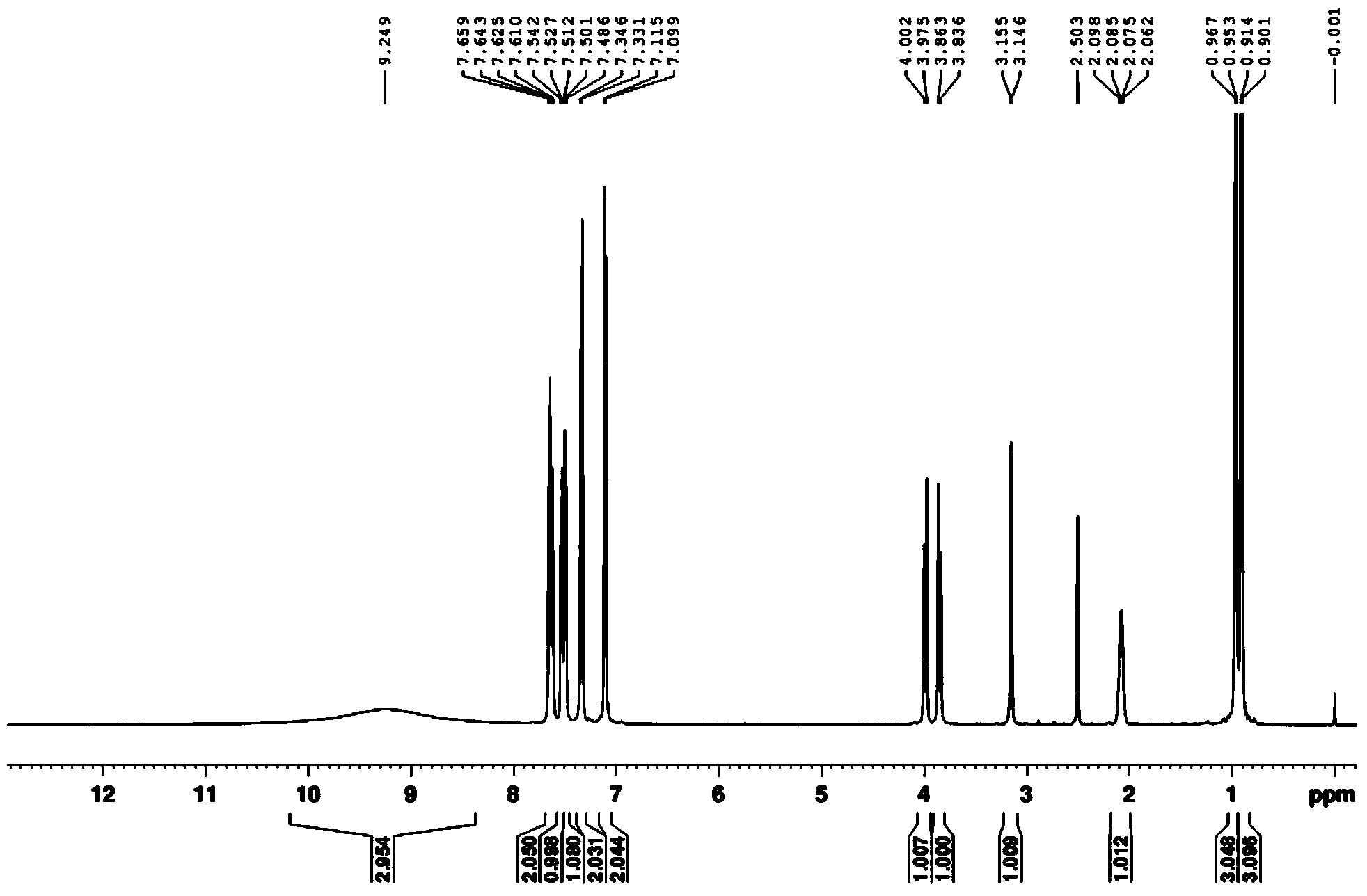
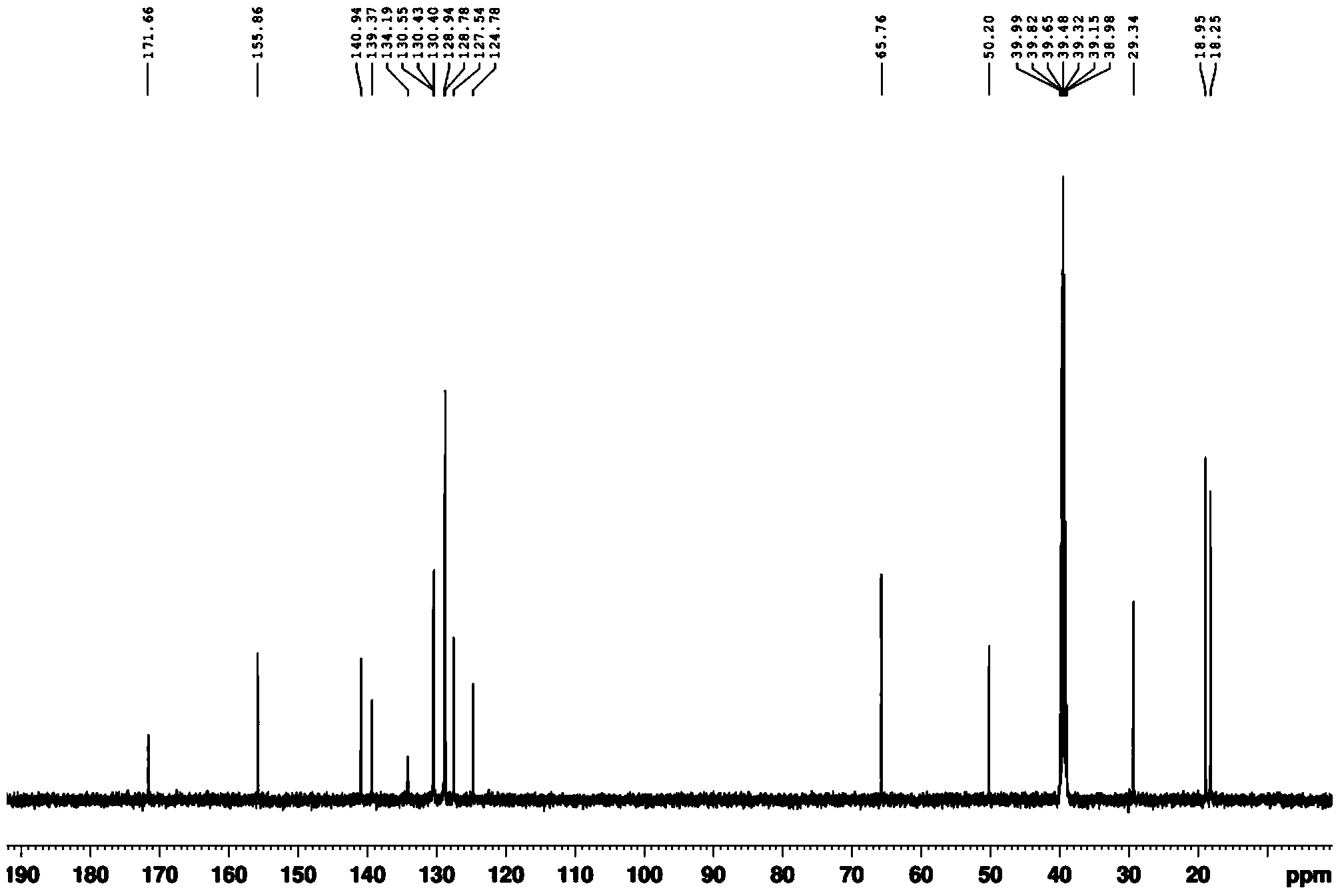
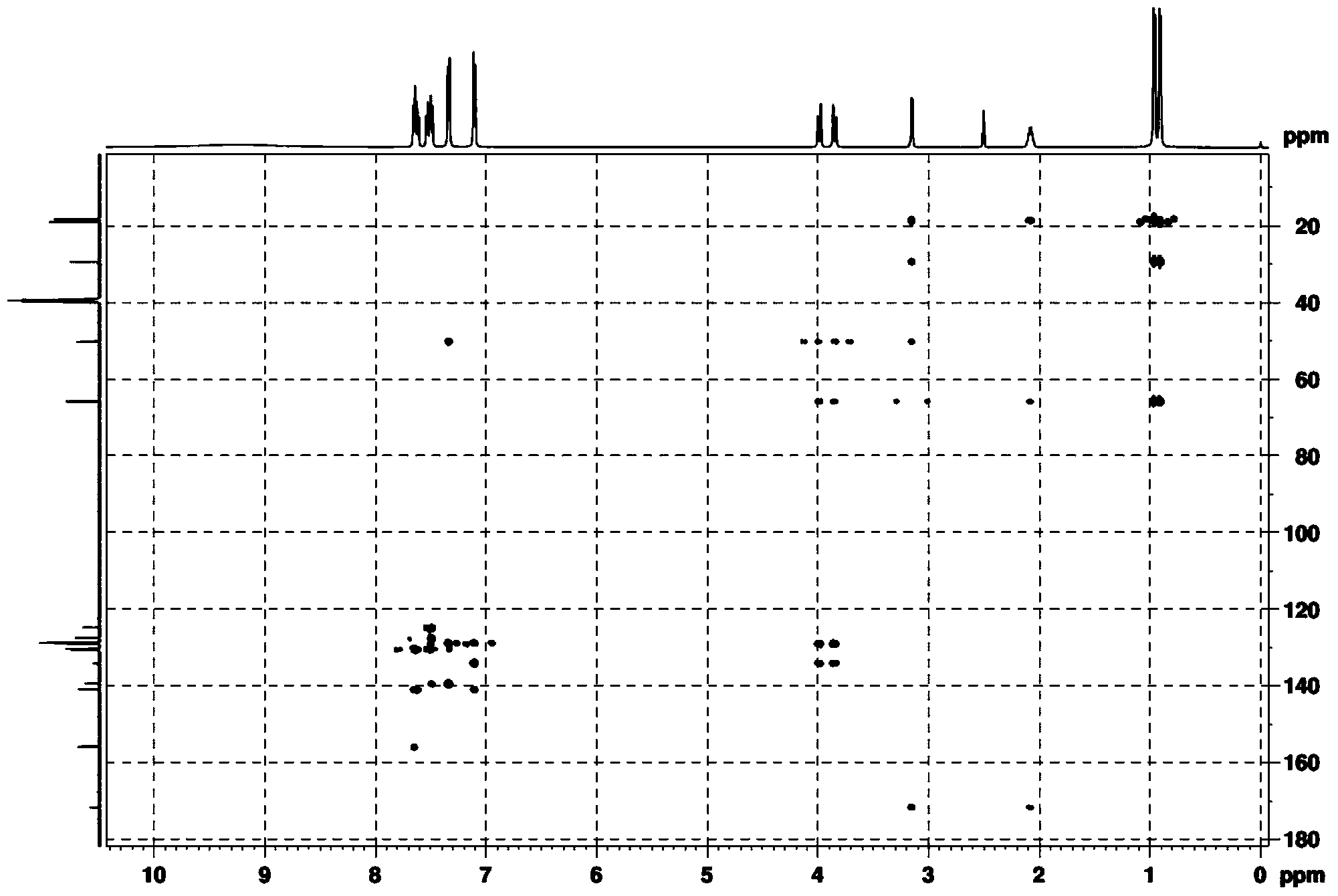
[0041] (2) Dry the white solid in a vacuum drying oven, then beat with 10 times the weight of water at room temperature for 2 hours, and filter with suction to obtain the pure product. Through DEPT spectrum, COZY spectrum, HMBC, 1 H-NMR spectrum, 13 The structure was confirmed by C-NMR spectrum, and the analyzed data are shown in Table 1.
Embodiment 2
[0042] Embodiment 2 Preparation of valsartan hydrolysis impurity compound
[0043] (1) Get 30g of valsartan, dissolve it in 200ml of acetonitrile, add 300ml of 4mol / L hydrochloric acid, heat and reflux for 5 hours, stir and drop to room temperature, then add 60% NaOH aqueous solution to adjust pH to neutral, separate phases, Add NaCl to the obtained aqueous phase to become a saturated solution, filter, extract the obtained saturated aqueous solution with n-butanol, take the organic phase, and evaporate to dryness to obtain a white solid;
[0044] (2) Dry the white solid in a vacuum drying oven, then beat with 8 times the weight of water at room temperature for 3 hours, and filter with suction to obtain the pure product. Through DEPT spectrum, COZY spectrum, HMBC, 1 H-NMR spectrum, 13 The structure was confirmed by C-NMR spectrum, and the analyzed data are shown in Table 1.
Embodiment 3
[0045] Embodiment 3 Preparation of valsartan hydrolysis impurity compound
[0046](1) Get 30g of valsartan, dissolve it in 200ml of acetonitrile, add 140ml of 9mol / L hydrochloric acid, heat and reflux for 3 hours, stir and drop to room temperature, then add 40% NaOH aqueous solution to adjust pH to neutral, separate phases, Add NaCl to the obtained aqueous phase to form a saturated solution, filter, extract the obtained saturated aqueous solution with n-butanol, take the organic phase, and evaporate to dryness to obtain a white solid;
[0047] (2) Dry the white solid in a vacuum drying oven, then beat with 12 times the weight of water at room temperature for 1 hour, and filter to obtain the pure product. Through DEPT spectrum, COZY spectrum, HMBC, 1 H-NMR spectrum, 13 The structure was confirmed by C-NMR spectrum, and the analyzed data are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com