Cerebrolysin hydrolysate injection and preparation method thereof
A technology for cerebroprotein hydrolysate and injection, which is applied in the field of cerebroprotein hydrolysate injection and its preparation, and achieves the effects of high guaranteed value, improved medication safety, and stable medicinal effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
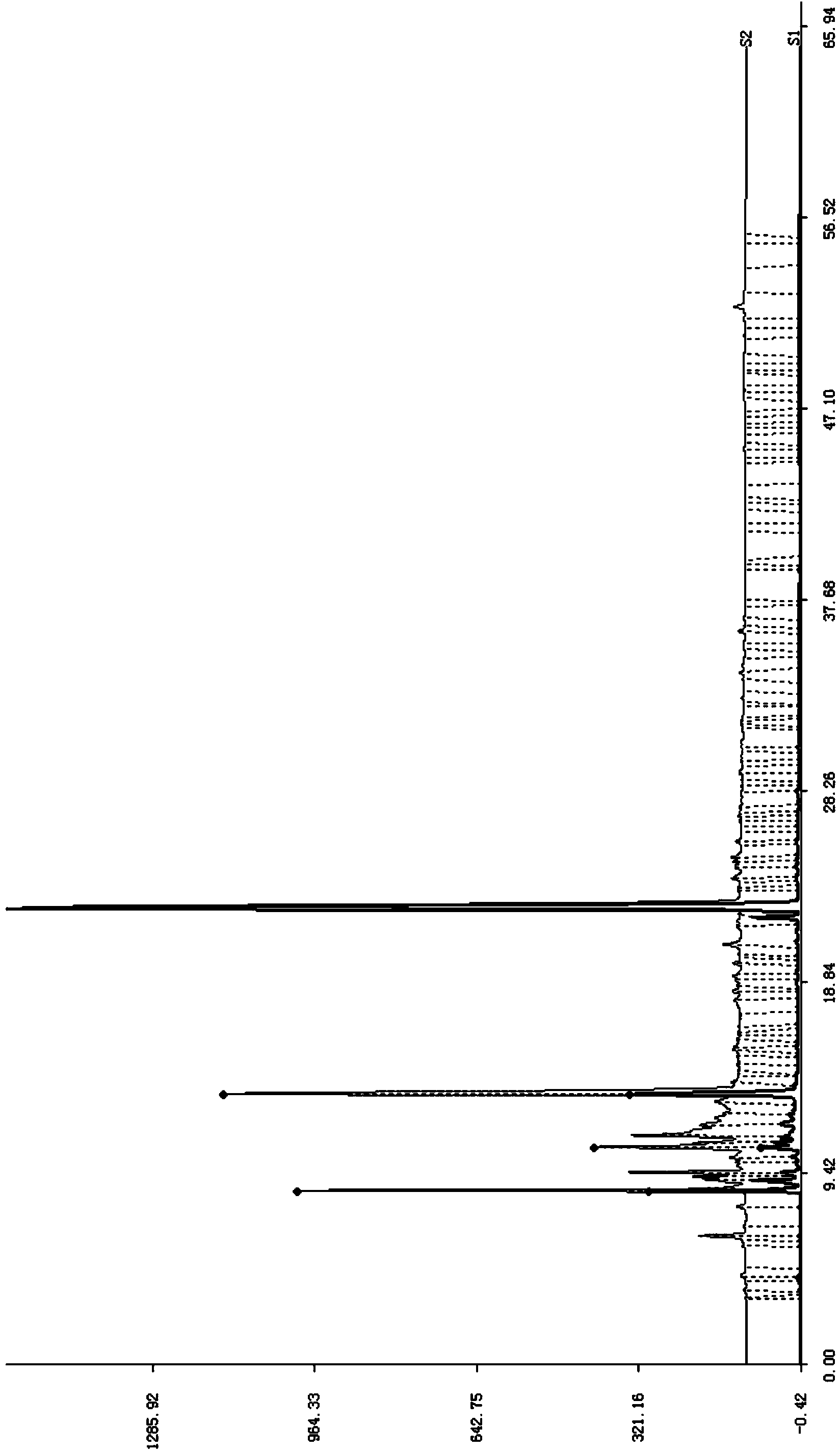
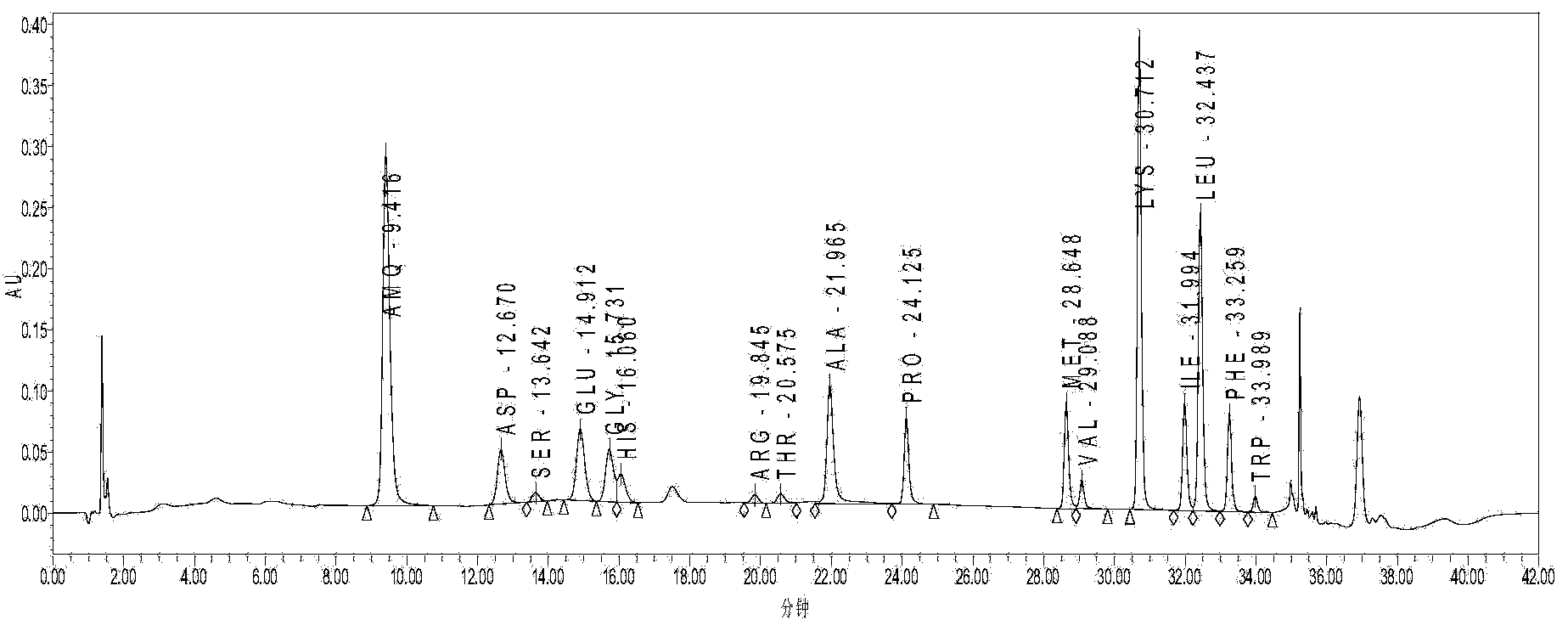
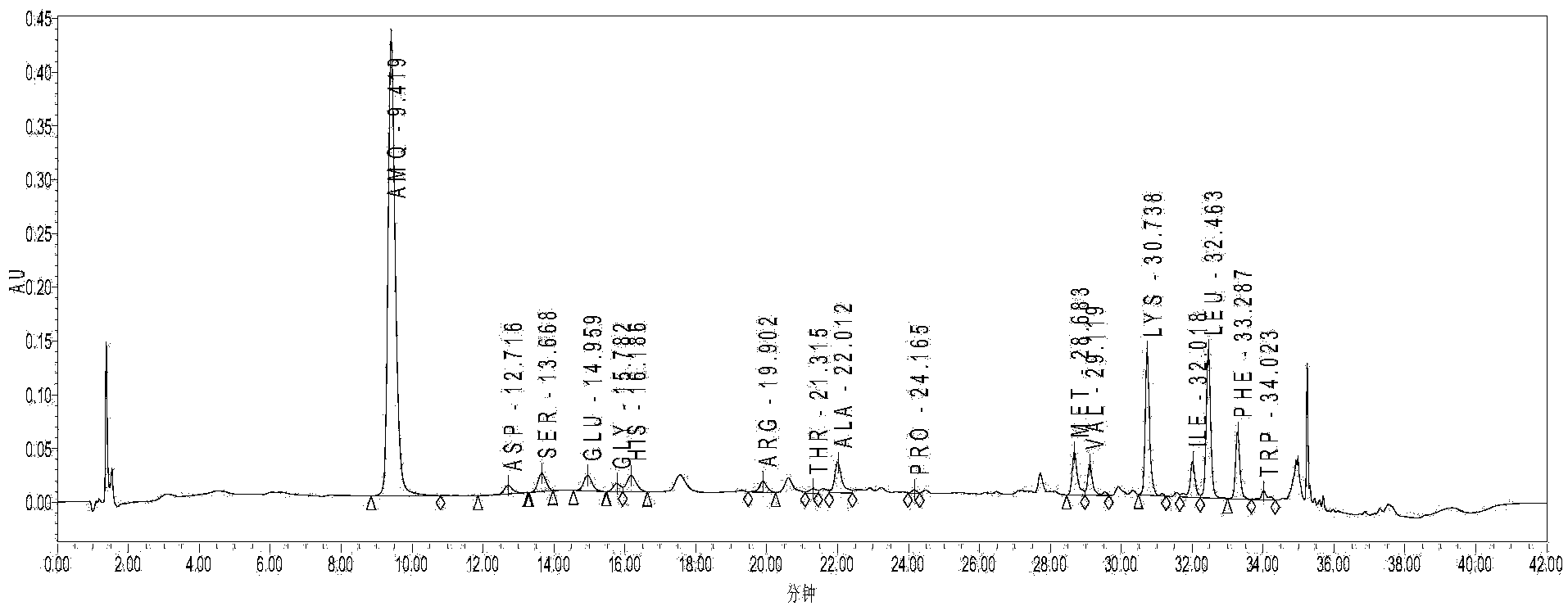
[0054] Take 100kg of brain tissue (quarantine qualified) after removing blood vessels, fat and other impurities, add 1 times the volume of purified water to homogenate, keep warm at 82°C for 15 minutes, cool; adjust pH to 1.8 with hydrochloric acid, hydrolyze with pepsin, keep warm at 42°C for 3 hours, Adjust the pH to 7.6 with sodium hydroxide, hydrolyze with trypsin, incubate at 42°C for 2 hours, and collect the supernatant by centrifugation; adjust the pH to 2 with hydrochloric acid, incubate at 105°C for 30 minutes, then adjust the pH to 8.8 with sodium hydroxide; cool down, extract the supernatant, 10kd membrane ultrafiltration to remove macromolecules, collect 100L of filtrate; apply column chromatography, wash column with purified water, analyze, collect eluate, adjust pH to 8.0-9.0, concentrate to 50L with DUS-8040C ion membrane, 0.2μm membrane Filtrate to obtain the stock solution, freeze and store below -15°C; thaw the stock solution, blend, add 0.01wt% sodium bisulfi...
Embodiment 2
[0069] Brain tissue (qualified for inspection and quarantine) removes impurities such as blood vessels and takes 100kg, add 2 times the volume of purified water to homogenate, keep warm at 85°C for 20 minutes, cool, adjust pH to 2.0 with hydrochloric acid, hydrolyze with pepsin, keep warm at 42°C for 4 hours, Adjust pH to 7.8 with sodium hydroxide, hydrolyze with trypsin, incubate at 42°C for 2 hours, collect supernatant by centrifugation; adjust pH to 2 with hydrochloric acid, incubate at 110°C for 30 minutes, adjust pH to 9.0 with sodium hydroxide; cool, extract supernatant, 10kd membrane Remove macromolecules by ultrafiltration, collect 150L of filtrate; apply column chromatography, wash column with purified water, analyze, collect eluate, adjust pH to 8.0-9.0, concentrate 50L with DUS-040C ion membrane, filter with 0.2μm membrane to obtain Stock solution, frozen and stored below -15°C; stock solution thawed, prepared, added 0.02wt% sodium bisulfite, adjusted to pH 6.9-7.5, ...
Embodiment 3
[0076] Brain tissue (qualified for inspection and quarantine) removes impurities such as blood vessels and takes 100kg, add 2 times the volume of purified water to homogenate, keep warm at 85°C for 30 minutes, cool, adjust pH to 3.0 with hydrochloric acid, hydrolyze with pepsin, keep warm at 35°C for 5 hours, Adjust the pH to 7.2 with sodium hydroxide, hydrolyze with trypsin, incubate at 45°C for 3 hours, and centrifuge to collect the supernatant; 10kd membrane ultrafiltration to remove macromolecules, collect 150L of filtrate; apply column chromatography, wash the column with purified water, analyze, collect eluate, adjust pH to 8.0-9.0, concentrate 50L with DUS-040C ion membrane, and filter with 0.2μm membrane , to obtain the original solution, and freeze it below -15°C; thaw the original solution, prepare it, add 0.02wt% sodium metabisulfite, adjust the pH to 6.9-7.5, 8-10kd membrane ultrafiltration, 0.2μm membrane filtration, filling; 115°C, 30 minutes , leak detection, et...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com