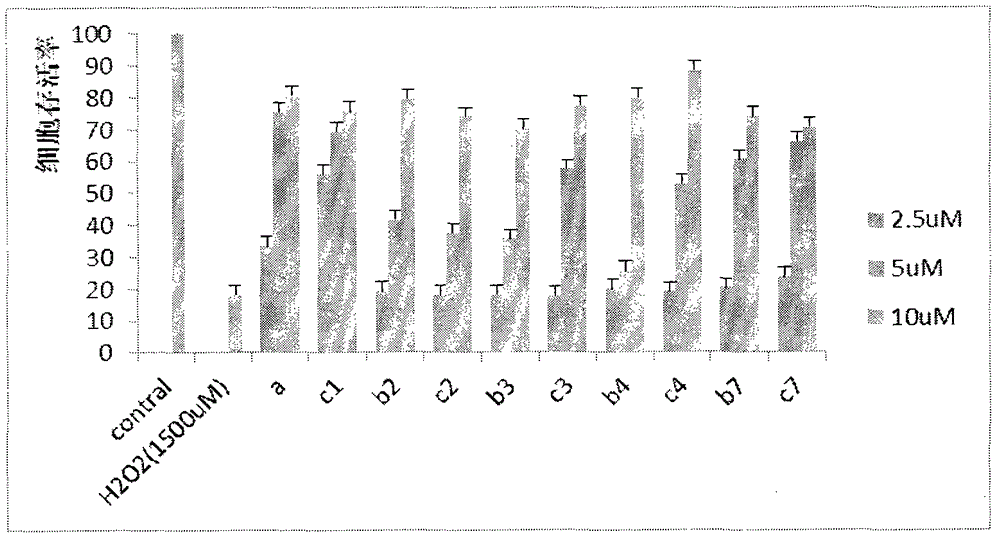
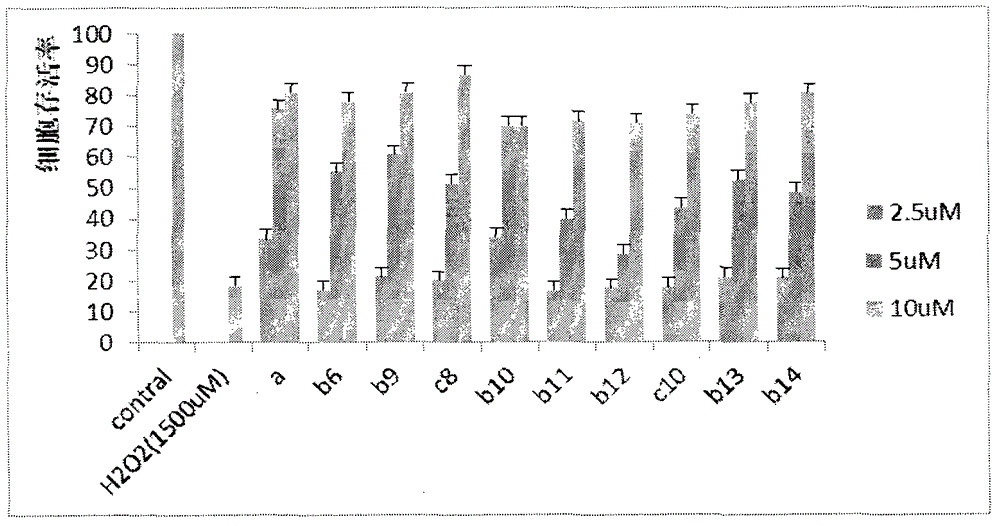
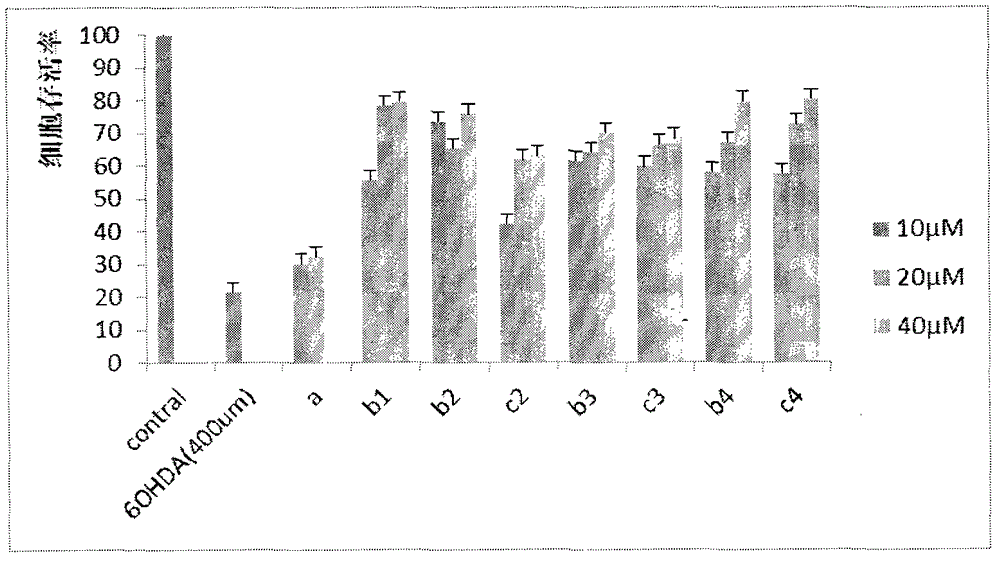
Preparation and application of caffeic acid ethyl benzene phenol hydroxyl protecting derivative taken as neuroprotective agent and antitumor medicine
A hydroxyl protecting group and phenylethyl alcohol ester technology, which is applied in the preparation of antineoplastic drugs, organic compounds, and carboxylic acid halides, can solve the problems of low bioavailability, strong gastric irritation, and insufficient antitumor activity. people's satisfaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Preparation of phenethyl caffeate (a)
[0034] Caffeic acid (9g, 50mmol) was added in batches to the reaction flask filled with 50ml DMSO, fully stirred, solid base potassium phosphate (12.74g, 60mmol) was added, fully stirred at room temperature, then slowly added dropwise bromoethylbenzene ( 7.48ml, 55mmol) of DMSO (20ml) solution into the reaction bottle, stirred at room temperature overnight, the reaction solution was dropped into 100ml of ice water, acidified with hydrochloric acid to pH 6, extracted with ethyl acetate, combined ester layer with saturated saline Washed, dried over anhydrous sodium sulfate, separated and purified by silica gel column (petroleum ether / ethyl acetate elution) to obtain white solid a, 11.36g, yield 80.0%, needle crystal, m.p., 102-103°C. 1 H-NMR (400MHz, DMSO) δ=9.21 (b, 2H, ArOH), 7.46 (d, J=16.0Hz, 1H, ArCH=CHCOO), 6.24 (d, J=16.0Hz, 1H, ArCH=CHCOO) , 6.75-7.34(m, 8H, ArH), 4.32(t, 2H, COOCH 2 R), 2.95(t, 2H, PhCH 2 R). 13 C-NMR (...
Embodiment 2
[0036] Preparation of (Z)-3-(3'-hydroxy-4'-acetoxyphenyl)-phenylethyl acrylate (b1)
[0037] Dissolve a (0.28g, 1mmol) in a reaction flask filled with 7ml of tetrahydrofuran, measure pyridine (0.08ml, 1mmol) with a pipette and add it dropwise to the reaction flask, then slowly add acetyl chloride (0.08g, 1mmol) of tetrahydrofuran (3ml) solution into the reaction flask, stirred at room temperature for 5h, TLC detection of complete reaction, spin-dried solvent under reduced pressure, added a small amount of water, extracted with ethyl acetate, the combined ester layer was washed with saturated brine, anhydrous sodium sulfate Dry, separate and purify with silica gel column (petroleum ether / ethyl acetate elution), and if necessary, separate and purify with thin-layer plate chromatography to obtain white solid b1, 0.08g, yield 24.5%, block crystal, m.p., 107- 108°C. 1 H-NMR (400MHz, CDCl 3 ) δ = 6.65 (s, 1H, ArOH), 7.55 (d, J = 16.0Hz, 1H, ArCH = CHCOO), 6.23 (d, J = 16.0Hz, 1H, ...
Embodiment 3
[0065] Preparation of (Z)-3-(3',4'-diacetoxyphenyl)-phenylethyl acrylate (c1)
[0066] Dissolve a (0.28g, 1mmol) in a reaction flask filled with 7ml of tetrahydrofuran, measure pyridine (0.32ml, 4mmol) with a pipette and add it dropwise to the reaction flask, then slowly add acetyl chloride (0.32g, 4mmol) of tetrahydrofuran (3ml) solution into the reaction flask, stirred at room temperature for 5h, TLC detection of complete reaction, spin-dried solvent under reduced pressure, added a small amount of water, extracted with ethyl acetate, the combined ester layer was washed with saturated brine, anhydrous sodium sulfate Dry, separate and purify with silica gel column (petroleum ether / ethyl acetate elution), if necessary, separate and purify with thin-layer plate chromatography to obtain white solid c1, 0.33g, yield 90.3%, needle crystal, m.p.82-83 ℃. 1 H-NMR (400MHz, CDCl 3 )δ=7.60(d, J=16.0Hz, 1H, ArCH=CHCOO), 6.36(d, J=16.0Hz, 1H, ArCH=CHCOO), 7.20-7.57(m, 8H, ArH), 4.41(t, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com