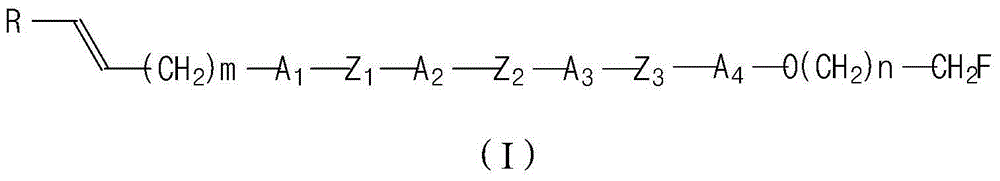Liquid crystal compound and its preparation method and use
A liquid crystal compound and selected technology, applied in the field of liquid crystal materials, can solve the problems of TFT-LCD such as insufficient fast response, low viscosity, and insufficient charge retention rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] Embodiment 1: intermediate Preparation of 4'-[2,3-difluoro-4-(2-fluoroethoxy)-phenyl]bicyclohexylcarbaldehyde (Compound 7)
[0084] 1) Synthesis of 2,3-difluoro-4-(2-fluoroethoxy)-bromobenzene (compound 2)
[0085]
[0086]Add 22mL of N,N-dimethylformamide into a 50mL reaction bottle, start stirring, add 5.2g of 2,3-difluoro-4-bromophenol (compound 1), after the solid is completely dissolved, add 10mL of water, 0.25g Tetrabutylammonium bromide, 4.0g anhydrous potassium carbonate. Heat up, control the temperature at 65-72°C, add 4.9g of 2-fluoroiodoethane dropwise, stir for 4 hours, add 10mL of toluene and 15mL of water, stir for 10 minutes, let stand to separate the liquid, and extract the water phase twice with 5mL×2 toluene (Stir for 2 minutes, let stand for 5 minutes), discard the water phase. All organic phases were combined and washed three times with 10 mL×3 water. The solvent was spin-dried and distilled under reduced pressure to obtain compound 2. Theor...
Embodiment 24
[0155] Synthesis of Example 24-[2,3-difluoro-4-(2-fluoroethoxy)-phenyl]-4'-vinyl-bicyclohexane (compound 8)
[0156]
[0157] Add 41.8g of methyl iodide phosphonium salt and 200mL of tetrahydrofuran into a 1L three-necked flask, cool down to minus 10°C, add 14.3g of potassium tert-butoxide, and react for 1 hour. Temperature controlled dropwise addition of 50mL tetrahydrofuran and 25.4g 4'-[2,3-difluoro-4-(2-fluoroethoxy)-phenyl]bicyclohexylformaldehyde (compound 7) mixed solution, natural heating reaction, stirring overnight , add 200mL of water, 100mL of ethyl acetate, stir, let stand to separate the liquid, concentrate the solvent, add 3 times the theoretical amount of petroleum ether, heat to 75 ° C, pass through a silica gel alumina heat column, concentrate the solvent to obtain 24.8g, add 2 times the theoretical amount of petroleum ether Hydro-ethanol recrystallization three times. Compound 8 was obtained, theoretical yield: 25.2 g, actual yield: 19.6 g, yield: 78.0%....
Embodiment 34
[0168] Example 3 Synthesis of 4'-(but-3-enyl)-4-[2,3-difluoro-4-(2-fluoroethoxy)-phenyl]bicyclohexane (compound 11)
[0169] 1) Synthesis of {4'-[2,3-difluoro-4-(2-fluoroethoxy)-phenyl]-bicyclohexyl}methanol (compound 9)
[0170]
[0171] A solution consisting of 77g of 4'-[2,3-difluoro-4-(2-fluoroethoxy)-phenyl]bicyclohexylformaldehyde (compound 7) and 200L of tetrahydrofuran in a 1L three-necked flask, temperature controlled at 0-10°C, 10.0 g of solid potassium borohydride was added in portions, and the addition was completed in about 30 minutes. Keep warm at 40-50°C for 3 hours. Control the temperature below 20°C, slowly add a solution consisting of 50mL of hydrochloric acid and 100mL of water dropwise, add 100mL of water and 100mL of dichloromethane into the reaction kettle, heat slightly to about 35°C, let stand to separate the liquid, and separate the lower product layer, The upper aqueous phase was extracted with 100 mL of dichloromethane, and the organic phases ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com