Diaryl substituted glycolythiourea compounds as well as preparation method and application thereof
A kind of technology of hydantoin thiourea and diaryl group, applied in the field of hydantoin thiourea compounds and preparation thereof, and can solve problems such as treatment failure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
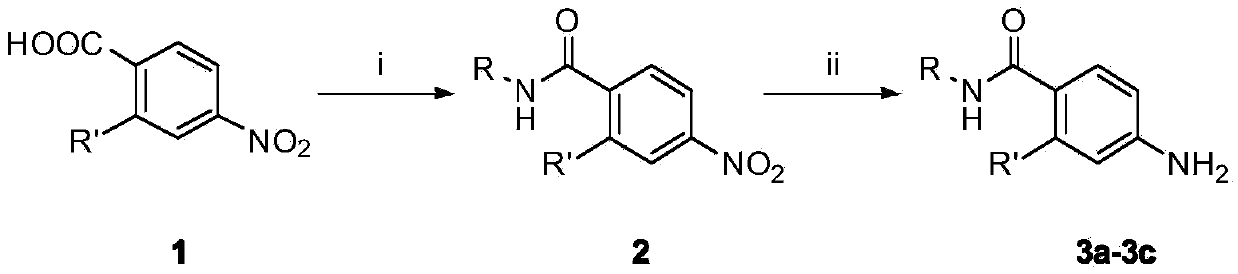
[0063] The synthesis of embodiment 1 substituted aniline 3a-3c
[0064] (1) Preparation of N-alkyl-2-substituted-4-nitrobenzamide
[0065] Put 2-substituted-4-nitrobenzoic acid (10mmol) in a 100mL reaction flask, add 30mL of anhydrous tetrahydrofuran, add 3 drops of DMF, stir well at room temperature, until the solid is completely dissolved, put the reaction flask in an ice alcohol bath , slowly add thionyl chloride (12mmol) dropwise, react at room temperature for 1h, evaporate the solvent under reduced pressure, dissolve the remaining solid with anhydrous DMF, and slowly add dropwise to 15ml of tetrahydrofuran solution of methylamine, ethylamine or isopropylamine (2mol / L), evaporate the organic solvent under reduced pressure, extract with ethyl acetate, combine the organic layers, wash with water, wash with saturated sodium chloride solution, dry over anhydrous sodium sulfate, filter, remove anhydrous sodium sulfate, evaporate under reduced pressure Solvent, to obtain interm...
Embodiment 2
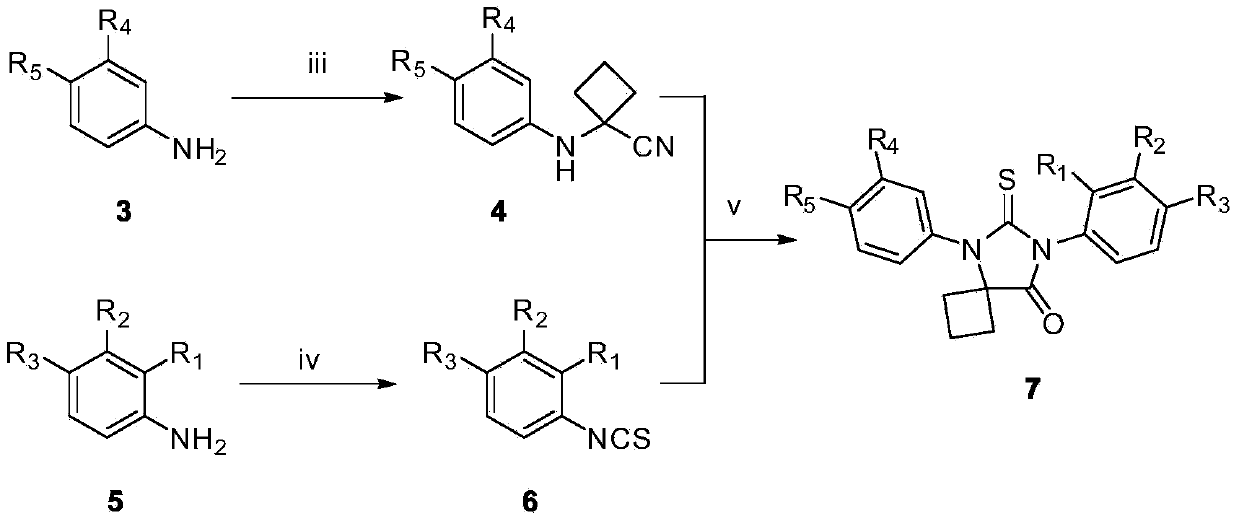
[0075] The preparation of embodiment 2 intermediate compound 4
[0076] Put the intermediate compound 3 (5mmol) and cyclobutanone (10mmol) in a 100mL two-neck flask, add 20mL of 90% glacial acetic acid to dissolve it completely, carefully add sodium cyanide (10mmol), quickly seal it, and reflux at 80°C for 24h. Pour the reaction mixture into 100 mL of ice water, adjust the pH value to neutral, extract with ethyl acetate, combine the organic phases, wash with water, wash with saturated sodium chloride solution, dry over anhydrous sodium sulfate, filter, and evaporate the solvent under reduced pressure to obtain The crude product was separated and purified by silica gel column chromatography to obtain intermediate 4, and the elution system was petroleum ether:ethyl acetate=3:1.
[0077] The intermediate compound 3 used was p-toluidine, p-fluoroaniline, N-isopropyl-4-aminobenzamide, N-ethyl-2-fluoro-4-aminobenzamide or N-isopropyl-2 -Fluoro-4-aminobenzamide, correspondingly, the...
Embodiment 3
[0083] The preparation of embodiment 3 intermediate 6 substituted phenyl isothiocyanates
[0084] C(S)Cl 2 (6.5mmol) and water (11mL) were added to a 25mL round-bottomed flask, stirred thoroughly at room temperature to form a heterogeneous system, and substituted aniline (compound 5) (6mmol) was added to the reaction solution, and reacted at room temperature for 1h to 2.5 h, the reaction solution was extracted with dichloromethane (15mL×3), the organic phases were combined and washed with saturated NaCl (30mL), and the organic phase was washed with anhydrous MgSO 4 After drying, filtering, and evaporating the solvent under reduced pressure, the intermediate compound 6 was separated by silica gel column chromatography, and the elution system was petroleum ether: ethyl acetate volume ratio 6:1.
[0085] The substituted aniline (compound 5) used above is 3-methoxy-4-cyanoaniline, 3-chloro-4-cyanoaniline, 3,4-dicyanoaniline, 2,3,4-trifluoroaniline, 3-Chloro-4-fluoroaniline, 3-br...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com