Preparation method of creatine phosphate disodium dihydrate
A technology for creatine disodium phosphate dihydrate and creatine, which is applied in the field of preparation of creatine phosphate disodium disodium dihydrate, can solve the problems of low yield and purity, long reaction time, low production efficiency and the like, and achieves purity High effect, short preparation time and high production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
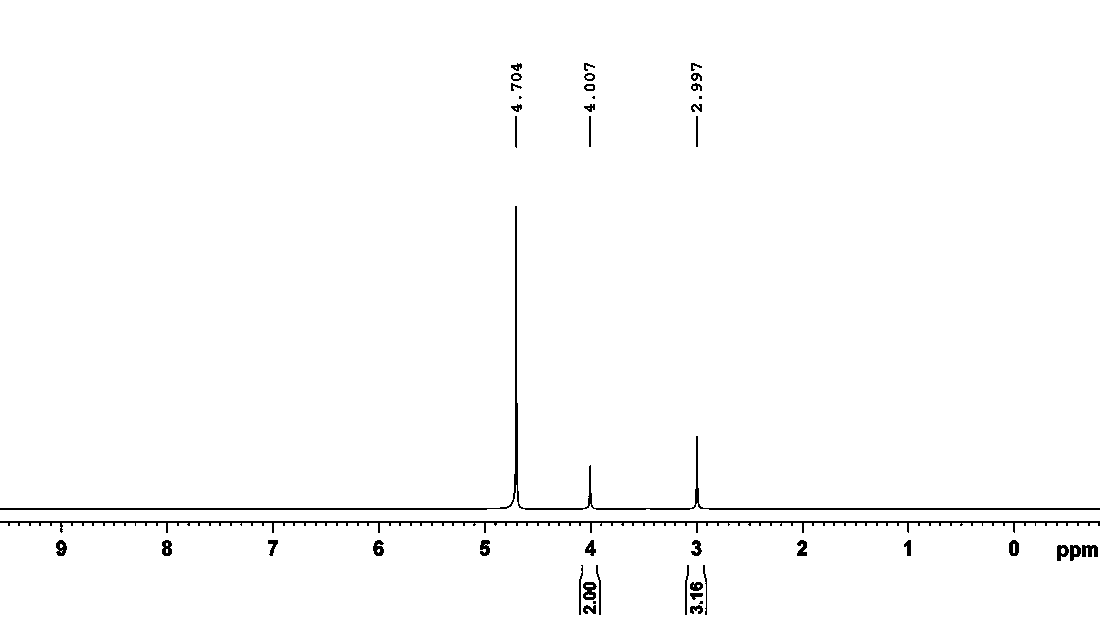
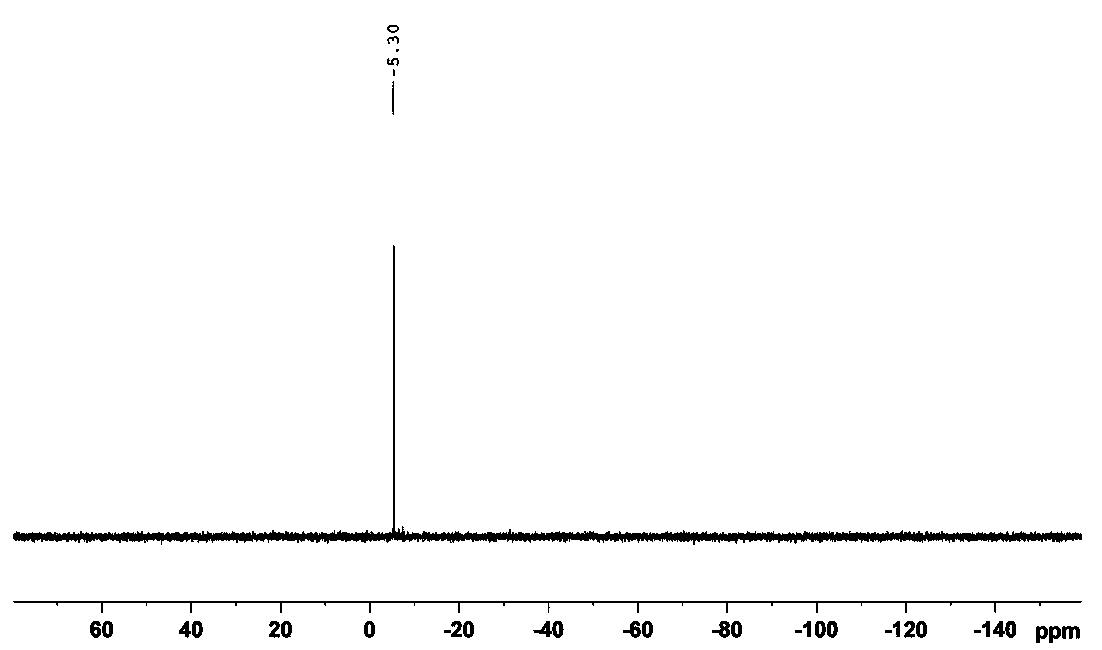
Image
Examples
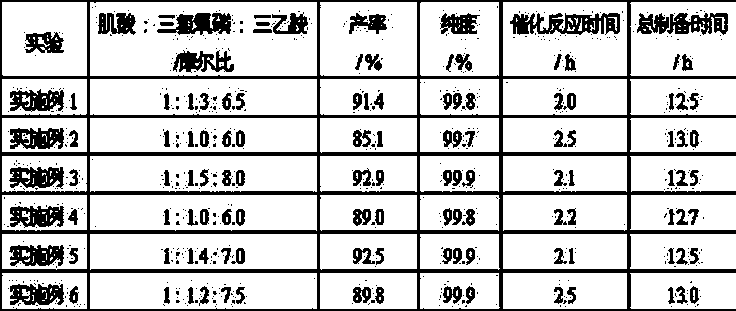
Embodiment 1
[0026] (1) Dissolve 131g (1 mol) of creatine in 500 mL of anhydrous acetonitrile, and gradually add 200g (1.3 mol) of phosphorus oxychloride in an ice bath, and then slowly add 656g (6.5 mol) of catalyst triethylamine; under the condition of -4°C-0°C, stir for 30 min to make it fully react, carry out the reaction, and obtain the reaction liquid;
[0027] (2) Add 54 mL (3mol) of water to the reaction solution, stir for 5 min, then add 1500 mL of absolute ethanol, and wait until the crystals are fully separated;
[0028] (3) Filtrate, dry the filter cake at 40°C, add 500 mL of ethanol aqueous solution with a concentration of 95% by volume, raise the temperature to 60°C to fully dissolve it, then let it stand at room temperature to fully separate out the crystals, filter, and use the same Wash the filter cake twice with an aqueous ethanol solution at a concentration of 40°C, and dry the filter cake under vacuum at 40°C to obtain pure creatine phosphate;
[0029] (4) Dissolve the...
Embodiment 2
[0031] (1) Dissolve 131g (1 mol) of creatine in 500 mL of anhydrous acetonitrile, and gradually add 154 g (1 mol) of phosphorus oxychloride under ice-bath conditions, after dissolving, slowly add 606 g (6.0 mol) catalyst triethylamine; under the condition of -4°C-0°C, stir for 1 h to make it fully react, and react to obtain a reaction solution;
[0032] (2) Add 54 mL (3mol) of water to the reaction solution, stir for 5 min, then add 1500 mL of absolute ethanol, and wait until the crystals are fully separated;
[0033] (3) Filtrate, dry the filter cake at 35°C, add 500 mL of ethanol aqueous solution with a concentration of 98% by volume, raise the temperature to 60°C to fully dissolve it, then let it stand at room temperature to fully separate the crystals, filter, and use volume Wash the filter cake once with an aqueous ethanol solution with a specific concentration of 98%, and vacuum-dry the filter cake at 40°C to obtain pure creatine phosphate;
[0034] (4) Dissolve the pur...
Embodiment 3
[0036](1) Dissolve 131 g (1 mol) of creatine in 500 mL of anhydrous acetonitrile, and gradually add 230 g (1.5 mol) of phosphorus oxychloride under ice bath conditions, and then slowly add 808 g ( 8.0 mol) of catalyst triethylamine; under the condition of -4°C-0°C, stir for 0.5 h to make it fully react, carry out the reaction, and obtain the reaction solution;
[0037] (2) Add 54 mL (3mol) of water to the reaction solution, stir for 5 min, then add 1500 mL of absolute ethanol, and wait until the crystals are fully separated;
[0038] (3) Filtrate, dry the filter cake at 40°C, add 500 mL of ethanol aqueous solution with a concentration of 90% by volume, raise the temperature to 60°C to fully dissolve, then let it stand at room temperature to fully separate out the crystals, filter, and use the same Wash the filter cake twice with an aqueous ethanol solution of a certain concentration, and dry the filter cake under vacuum at 45°C to obtain pure creatine phosphate;
[0039] (4) ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com