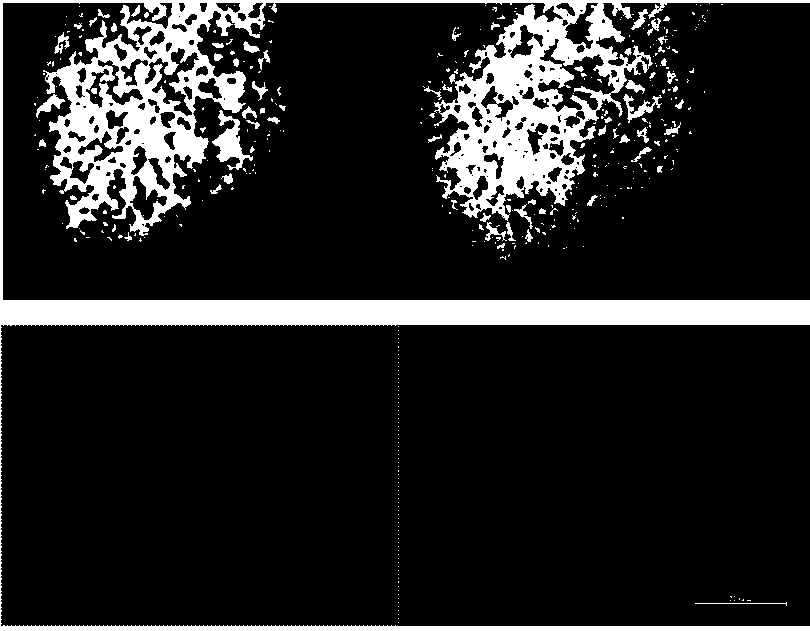Isoquinoline compounds capable of inhibiting inhibitor-of-apoptosis proteins and preparation method and application of isoquinoline compounds
A technology of apoptosis-inhibiting proteins and compounds, applied in the field of isoquinoline compounds and their preparation, can solve the problems of inability to inhibit IAP proteins, low bioavailability, and short action time, and achieve broad action objects and pathways and anti-tumor activity The effect of high and small molecular mass
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1 CCK-8 method detects cell viability and confirms the compound 1 For ovarian cancer A2780, A2780cisR has an anti-tumor effect, see figure 1 shown. By CCK-8 method, we tested the cell viability of A2780 and A2780cisR cells treated with compound 1. It was found that compound 1 can inhibit the proliferation of ovarian cancer cells, and this inhibitory effect is concentration-dependent. Among them, at a concentration of 10 μg / mL, the tumor inhibition rate of the cells was 69.8%. At the same time, according to the tumor cell growth inhibition curve, the IC50 of compound 1 against A2780 and A2780cisR were 5.9 and 64.4 μg / mL, respectively.
Embodiment 2
[0052] Example 2 Detection of cell apoptosis rate by Ho chest staining to confirm compound 1 It can promote the apoptosis of ovarian cancer SKOV3. See figure 2 shown. The apoptotic ability of ovarian cancer SKOV3 treated with Compound 1 was analyzed by Hochest staining. Among them, compared with the blank control group, the number of apoptotic cells in the ovarian cancer A2780 and A2780cisR cells treated with compound 1 increased, and the number was B01002 (246.3±35.6) and (125.7±34.9) in turn, compared with the blank control group There were statistical differences (P figure 2 It can be seen that many obvious nuclear fragmentation and nuclear pyknosis can be seen in the ovarian cancer cells treated with compound 1.
Embodiment 3
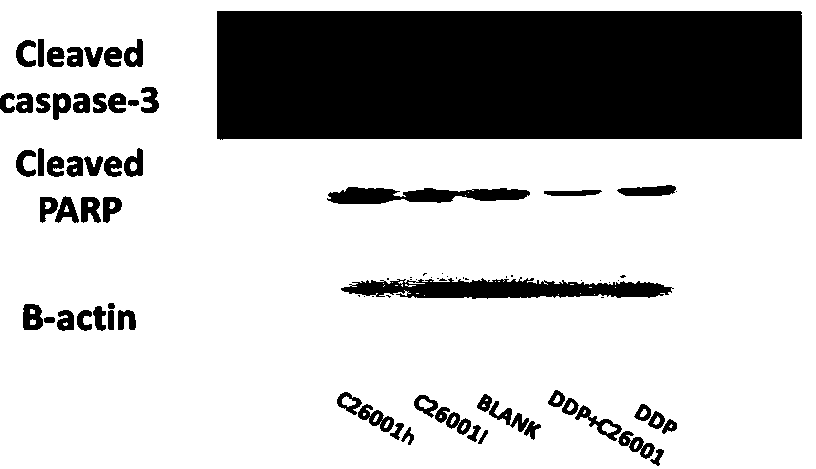
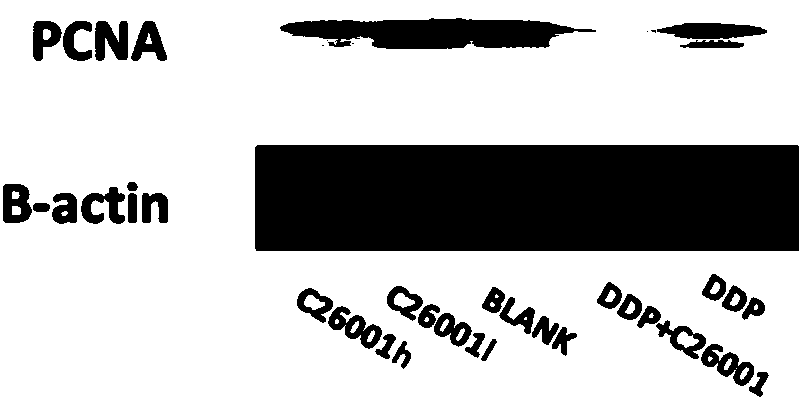
[0053] Example 3 The expression of PARP, Caspase-3, and PCNA was detected by Western Blot, and it was confirmed that compound 1 has the effect of promoting apoptosis and inhibiting proliferation of ovarian cancer SKOV3 cells, see image 3 , Figure 4 shown. Caspase-3 is a key protein in the IAP protein inhibiting apoptosis signaling pathway, so we used Western Blot method to detect the expression of Caspase-3 in each group of tumor tissues, and we also performed the activation of the Caspase cleavage substrate PARP Protein—determination of the expression of cleaved PARP protein. The results showed that the expressions of cleaved PARP and cleaved Caspase-3 were different in each group, and the expression of cleaved PARP and cleaved Caspase-3 in the tumor tissue of the nude mice in the compound 1 group was the highest. In addition, the inhibition of tumor growth by compound 1 was reflected by detecting the expression of PCNA in tumor tissue. The results showed that the express...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com