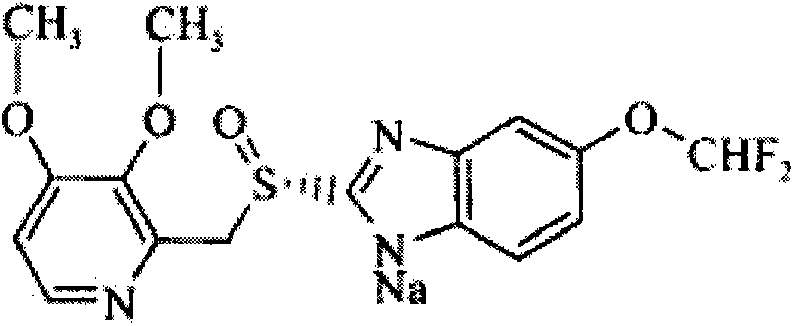
S-pantoprazole sodium enteric-coated tablet and preparation method thereof
A technology of pantoprazole sodium and enteric-coated tablets, which is applied in the direction of pharmaceutical formulas, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problems of large coefficient of variation and poor stability, and achieve stability Excellent performance, small coefficient of variation, good curative effect and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
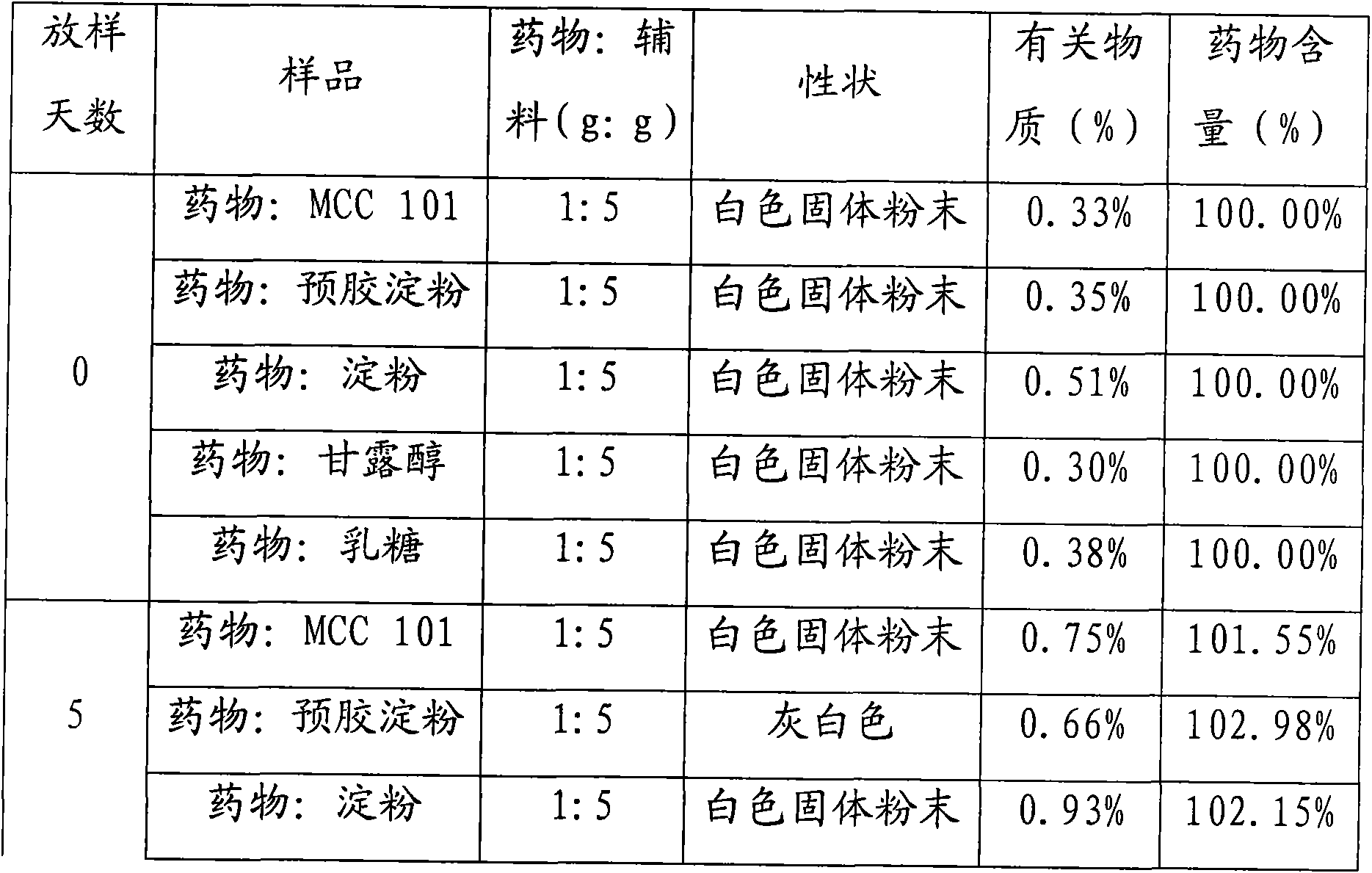
Image
Examples
Embodiment 1
[0023] The preparation of embodiment one S-pantoprazole sodium enteric-coated tablet
[0024] 1. Chip core
[0025] Material name
Dosage
S-pantoprazole sodium sesquihydrate
22.55mg
27mg
63mg
Cross-linked polyvinylpyrrolidone
4mg
7mg
2% hypromellose 50% ethanol solution
0.6mg
0.91mg
[0026] 2. Isolation layer
[0027] Opadry 17K69000
12g
double distilled water
88g
[0028] 3. Enteric-coated layer
[0029] Opadry 93O36628
20g
double distilled water
80g
[0030] The preparation of tablet core: get the S-pantoprazole sodium sesquihydrate of recipe quantity, microcrystalline cellulose, mannitol, cross-linked polyvinylpyrrolidone, magnesium oxide, cross 80 mesh sieves and mix; Add 2 50% ethanol solution of % hypromellose, passed through a 20-mesh sie...
Embodiment 2
[0034] The preparation of embodiment two S-pantoprazole sodium enteric-coated tablets
[0035] 1. Chip core
[0036] Material name
Dosage
S-pantoprazole sodium sesquihydrate
22.55mg
45mg
45mg
8mg
7mg
2% hypromellose 50% ethanol solution
0.6mg
0.91mg
[0037] 2. Isolation layer
[0038] Opadry 17K69000
12g
double distilled water
88g
[0039] 3. Enteric-coated layer
[0040] Opadry 93O36628
20g
double distilled water
80g
[0041] The preparation of tablet core: get the S-pantoprazole sodium sesquihydrate of recipe quantity, microcrystalline cellulose, mannitol, sodium carboxymethyl starch, magnesium oxide, cross 80 mesh sieves and mix; Add 2 50% ethanol solution of % hypromellose, passed through a 20-mesh sieve to granu...
Embodiment 3
[0045] The preparation of embodiment three S-pantoprazole sodium enteric-coated tablets
[0046] 1. Chip core
[0047] Material name
Dosage
S-pantoprazole sodium sesquihydrate
22.55mg
27mg
63mg
8mg
magnesium oxide
7mg
2% hypromellose 50% ethanol solution
0.6mg
0.91mg
[0048] 2. Isolation layer
[0049] Opadry 17K69000
12g
double distilled water
88g
[0050] 3. Enteric-coated layer
[0051] Opadry 93O36628
20g
double distilled water
80g
[0052] The preparation of tablet core: get the S-pantoprazole sodium sesquihydrate of recipe quantity, microcrystalline cellulose, mannitol, sodium carboxymethyl starch, magnesium oxide, cross 80 mesh sieves and mix; Add 2 50% ethanol solution of % hypromellose, passed through a 20-mesh sieve to gra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com