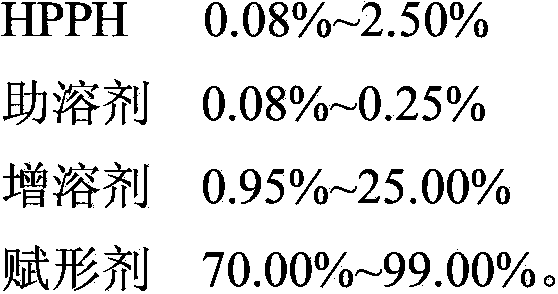Freeze-dried HPPH powder injection preparation for injection and preparation method thereof
A technology for freeze-dried powder injection and injection, which is applied in the field of medicine, can solve the problems of the safety and effectiveness of clinical medication, the unfavorable production of water injection, and the poor stability, etc., and achieves low dark toxicity, loose preparation, and formulation. reasonable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029]
[0030] a) Move the mannitol of the prescribed amount to the batching tank equipped with 2000ml water for injection, stir and dissolve to obtain the solution ①;
[0031] b) Weigh the prescribed amount of HPPH and place it in the batching tank, add the prescribed amount of absolute ethanol, stir, add the prescribed amount of sodium carbonate solution and ELP, stir and dissolve to obtain the solution ②;
[0032] c) Add solution ② to solution ①, stir, add water for injection to 5000ml, then add phosphoric acid aqueous solution dropwise to adjust the pH of the solution to 7.0, filter and sterilize to the liquid storage tank, subpackage, semi-tampon, freeze-dry, and plug ,Package. Wherein the freeze-drying procedure is as follows:
[0033] 1) Pre-freezing: Pre-freeze the product at -40~-50°C for 3 hours to freeze the product until solid;
[0034] 2) Primary drying: After the product is pre-frozen, turn on the vacuum pump to evacuate to 20-30 Pa, raise the temperature o...
Embodiment 2
[0037]
[0038] a) Move the dextran of prescription quantity to the batching tank that 2500ml water for injection is housed, stir and dissolve to obtain solution ①;
[0039] b) Take the HPPH of the recipe quantity and put it in the batching tank, add the 96% ethanol of the recipe quantity, stir, add the sodium carbonate solution and Tween 80 of the recipe quantity, stir and dissolve to obtain the solution ②;
[0040] c) Add solution ② to solution ①, stir, add water for injection to 5000ml, then add phosphoric acid aqueous solution dropwise to adjust the pH of the solution to 6.5, filter and sterilize to the liquid storage tank, subpackage, semi-tampon, freeze-dry, and stopper ,Package. Wherein the freeze-drying procedure is as follows:
[0041] 1) Pre-freezing: Pre-freeze the product at -40~-50°C for 3.5 hours to freeze the product until solid;
[0042] 2) Primary drying: After the product is pre-frozen, turn on the vacuum pump to evacuate to 20-30 Pa, raise the temperatu...
Embodiment 3
[0045]
[0046] a) Move the prescribed amount of mannitol into a batching tank equipped with 3000ml water for injection, stir and dissolve to obtain solution ①;
[0047] b) Weighing the HPPH of the prescription quantity and placing it in the batching tank, adding 95% ethanol of the prescription quantity, stirring, adding the sodium carbonate solution and polyethylene glycol stearate 15 of the prescription quantity, stirring and dissolving to obtain the solution ②;
[0048] c) Add solution ② to solution ①, stir, add water for injection to 5000ml, add phosphoric acid aqueous solution dropwise to adjust the pH of the solution to 7.8, filter and sterilize to the liquid storage tank, sub-package, semi-tampon, freeze-dry, and stopper, Package. Wherein the freeze-drying procedure is as follows:
[0049] 1) Pre-freezing: Pre-freeze the product at -40~-50°C for 4 hours to freeze the product until solid;
[0050] 2) Primary drying: After the pre-freezing of the product, turn on the...
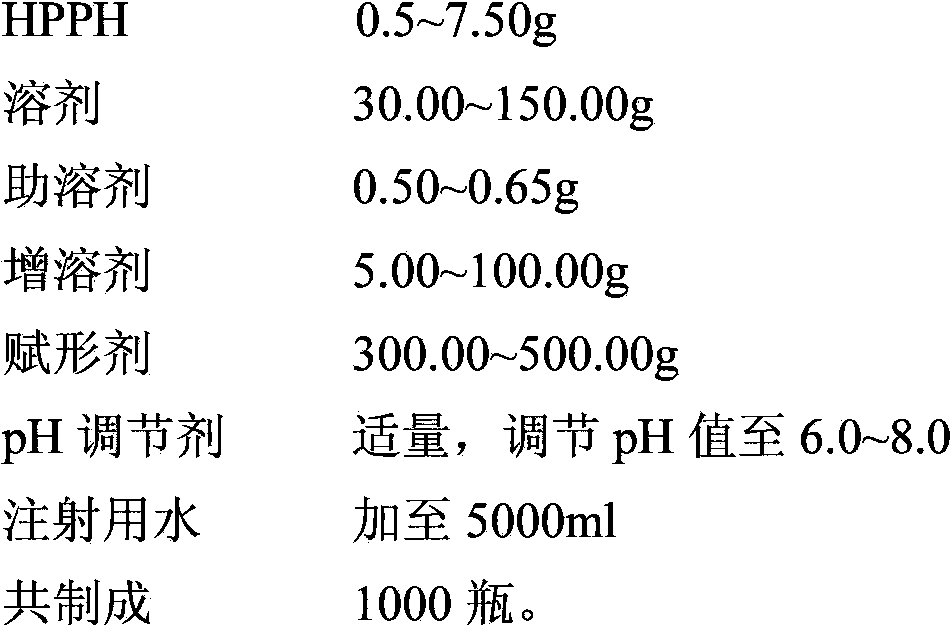
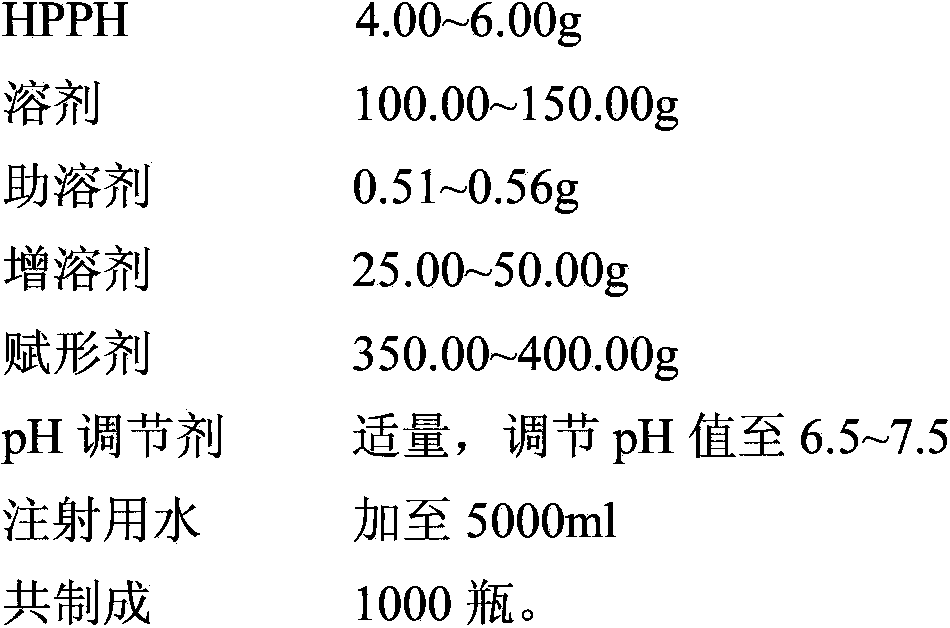
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com