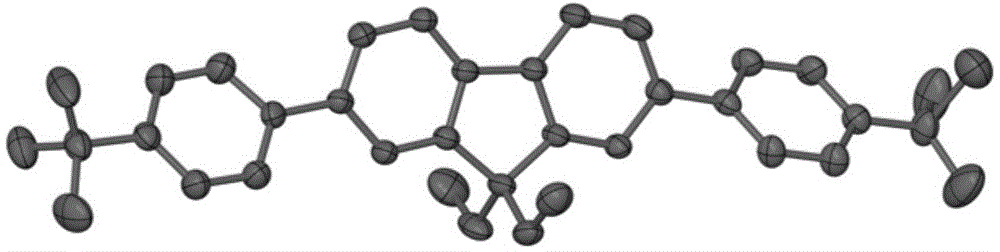
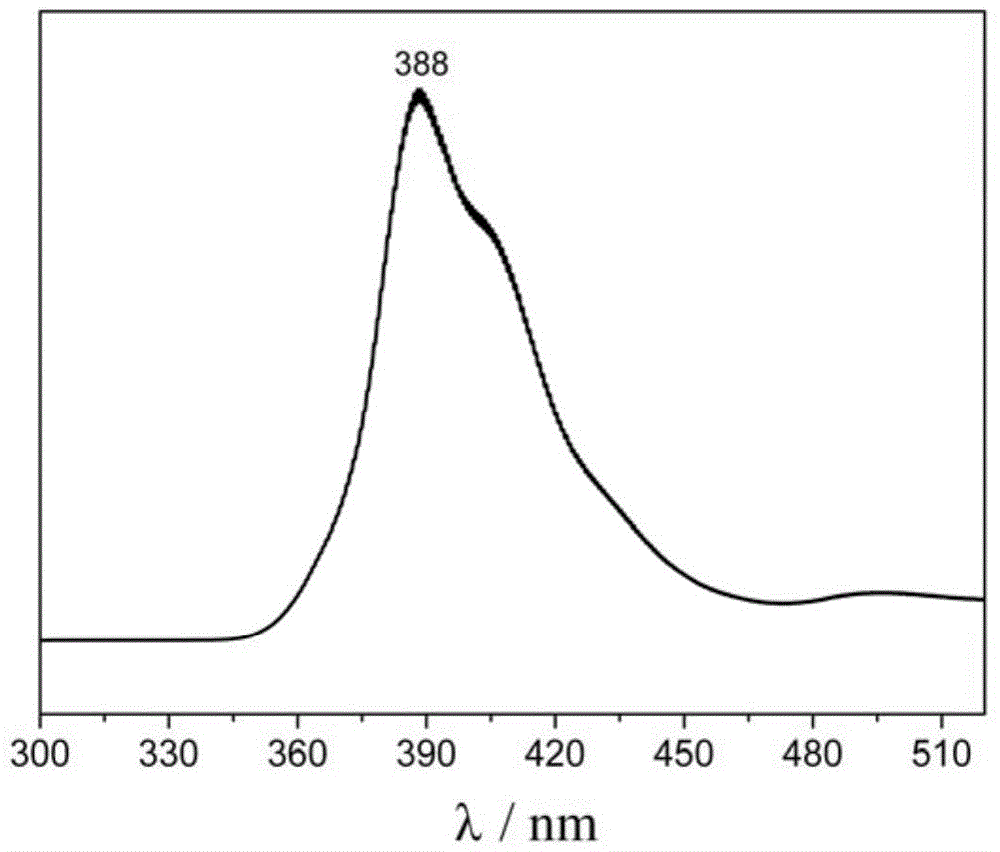
Purple fluorescent material containing tertiary butyl fluorene
A technology of violet fluorescence and tert-butyl fluorene is applied in the field of fluorescent materials to achieve the effects of increasing electron mobility, increasing electron cloud density, and improving luminous intensity and stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Pd(PPh 3 ) 4 (0.069g, 0.06mmol) and potassium carbonate (0.829g, 6mmol) were placed in a 150mL three-necked round-bottomed flask, and 40mL of toluene, 30mL of ethanol and 10mL of water were added; the three-necked round-bottomed flask was evacuated and filled with nitrogen, and repeated Operated 3 times; the mixture was refluxed at 80°C for 2 days under the protection of nitrogen; after the reaction, cooled and separated, the aqueous phase was extracted with dichloromethane, the organic phase was combined, and the organic phase was washed with saturated brine for 3 Afterwards, use magnesium sulfate to dry, filter to get the filtrate; spin dry the solvent in vacuo to get the solid powder of the crude product, then use petroleum ether as eluting agent and adopt column chromatography to separate and purify to obtain 2,7-bis(4-tert-butyl) Phenyl-9,9-diethylfluorene.
Embodiment 2
[0023] Take 2,7-dibromo-9,9-diethylfluorene (0.648g, 2mmol), 4-tert-butylphenylboronic acid (1.068g, 6mmol), Pd(PPh 3 ) 4 (0.231g, 0.2mmol) and potassium carbonate (1.659g, 12mmol) were placed in a 250mL three-necked round-bottomed flask, and 80mL of toluene, 70mL of ethanol and 10mL of water were added; the three-necked round-bottomed flask was evacuated and filled with nitrogen, and repeated Operation 3 times; the mixture was refluxed at 85°C for 3 days under the protection of nitrogen; after the reaction, the liquid was cooled and separated, the aqueous phase was extracted with dichloromethane, the organic phase was combined, and the organic phase was washed with saturated brine for 2 Afterwards, use magnesium sulfate to dry, filter to get the filtrate; spin dry the solvent in vacuo to get the solid powder of the crude product, then use petroleum ether as eluting agent and adopt column chromatography to separate and purify to obtain 2,7-bis(4-tert-butyl) Phenyl-9,9-diethyl...
Embodiment 3
[0025] Take 2,7-dibromo-9,9-diethylfluorene (0.6479g, 2mmol), 4-tert-butylphenylboronic acid (1.424g, 8mmol), Pd(PPh 3 ) 4 (0.092g, 0.08mmol) and potassium carbonate (2.764g, 20mmol) were placed in a 250mL three-necked round-bottomed flask, and 60mL of toluene, 40mL of ethanol and 10mL of water were added; the three-necked round-bottomed flask was evacuated and filled with nitrogen, and repeated Operated 3 times; the mixture was refluxed at 90°C for 4 days under the protection of nitrogen; after the reaction, cooled and separated, the aqueous phase was extracted with dichloromethane, and the organic phase was combined, and then the organic phase was washed with saturated brine 2 times, then dried with magnesium sulfate, filtered to get the filtrate; the solvent was spin-dried in vacuo to obtain the solid powder of the crude product, and then separated and purified by column chromatography with petroleum ether as eluent to obtain 2,7-bis(4-tert-butyl ) phenyl-9,9-diethylfluore...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com