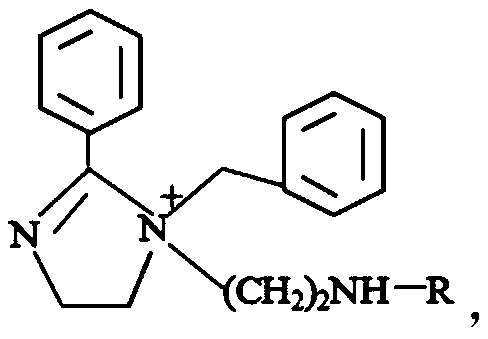
A kind of thiourea-modified imidazoline derivative corrosion inhibitor and its preparation method and application
A technology of imidazoline and derivatives is applied in the field of thiourea-based modified imidazoline derivative corrosion inhibitor and its preparation field, which can solve the problems of poor film-forming property, few electron-donating bases, unsuitable corrosion, etc. The effect of fluidity, low toxicity and good corrosion inhibition effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] This example provides a preparation method of a thiourea-modified imidazoline derivative corrosion inhibitor. The thiourea-modified imidazoline is obtained by reacting benzoic acid and diethylenetriamine to obtain an imidazoline intermediate, reacting with benzyl chloride to obtain an imidazoline quaternary ammonium salt, and finally reacting with thiourea.
[0036] The present embodiment provides the specific synthesis steps of the above-mentioned modified imidazoline, and the specific steps are as follows:
[0037] Add benzoic acid and diethylenetriamine into the container. Benzoic acid (1mol) and diethylenetriamine (1.3mol) are in the presence of the water-carrying agent xylene, and the temperature is gradually raised, first at 100°C, and reacted for 2h; Then raise the temperature to 210°C for ring formation reaction, 4h, to obtain the imidazoline intermediate; cool to 80°C, slowly add benzyl chloride (1.2mol) dropwise, carry out quaternization reaction, 2 ~ 5h; fina...
Embodiment 2
[0040] This example provides a preparation method of a dithiourea-modified imidazoline derivative corrosion inhibitor. The dithiourea-modified imidazoline is obtained by reacting benzoic acid and diethylenetriamine to obtain an imidazoline intermediate, reacting with benzyl chloride to obtain an imidazoline quaternary ammonium salt, and finally reacting with thiourea.
[0041] The present embodiment provides the specific synthesis steps of the above-mentioned modified imidazoline, and the specific steps are as follows:
[0042]Add benzoic acid and diethylenetriamine into the container. Benzoic acid (1mol) and diethylenetriamine (1.3mol) are in the presence of the water-carrying agent xylene, and the temperature is gradually raised, first at 100°C, and reacted for 2h; Then raise the temperature to 210°C for ring formation reaction, 4h, to obtain the imidazoline intermediate; cool to 80°C, slowly add benzyl chloride (1.2mol) dropwise, carry out quaternization reaction, 2 ~ 5h; f...
Embodiment 3
[0045] This example provides a preparation method of a phenylthiourea-modified imidazoline derivative corrosion inhibitor. The phenylthiourea-modified imidazoline is obtained by reacting benzoic acid and diethylenetriamine to obtain an imidazoline intermediate, then reacting with benzyl chloride to obtain an imidazoline quaternary ammonium salt, and finally reacting with phenylthiourea.
[0046] This embodiment provides the specific synthesis steps of the above-mentioned phenylthiourea-modified imidazoline, and the specific steps are as follows:
[0047] Add benzoic acid and diethylenetriamine into the container. Benzoic acid (1mol) and diethylenetriamine (1.3mol) are in the presence of the water-carrying agent xylene, and the temperature is gradually raised, first at 100°C, and reacted for 2h; Then heat up to 210°C for ring formation reaction, 4h, to obtain the imidazoline intermediate; cool to 80°C, slowly add benzyl chloride (1.2mol) dropwise, carry out quaternization react...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com