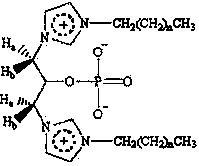
1,1'-dialkyl-3,3'-(2-phosphate-1,3-propylidene)imidazolium compound and preparation method thereof
A technology of alkyl imidazole and alkyl benzimidazole, which is applied in the field of 1,1'-dialkyl-3,3'-imidazole inner salt compound and its preparation, can solve problems such as difficult synthesis, and reduce corrosiveness , The synthesis method is simple, and the water solubility is good.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] The first step: the synthesis of 1,3-dichloro-2-propanol
[0038] Add 30.0 g of 36% concentrated HCl to a three-necked flask equipped with a stirrer and a thermometer, and then add 24.5 g of epichlorohydrin dropwise, and the dropwise addition is completed in about half an hour, and the temperature is controlled between 30°C and 40°C. About 7~9 h of reaction. Separation was carried out with a separatory funnel to obtain the organic phase, and the organic phase was separated with anhydrous MgSO 4 Dry for about 4-5 h, filter to remove magnesium sulfate, and distill under normal pressure to collect fractions at 175°C-177°C to obtain 23.9 g of colorless liquid 1,3-dichloro-2-propanol with a yield of 70.0%.
[0039] 1,3-Dichloro-2-propanol 1 H NMR (400 MHz, DMSO-d 6 ) δ H : 3.15 (1H, d, J = 6.4 Hz, -OH), 3.70 (4H, d, J = 5.6 Hz, 2-CH 2 Cl), 4.08 (1H, m, -CH(OH)-).
[0040] The second step: the synthesis of N-octylimidazole
[0041] Add 0.440 g (11.0 mmol) NaO...
Embodiment 2
[0050] The second step: the synthesis of N-decyl imidazole
[0051] Add 0.440 g (11.0 mmol) NaOH, 0.714 g (10.5 mmol) imidazole and 10 mL dimethylsulfoxide (DMSO) into a three-necked flask equipped with a stirrer and a thermometer, and stir at 20°C to 25°C under nitrogen protection until To the transparent solution, 2.21 g (10.0 mmol) of decane bromide was added dropwise, and after about 4~6 h of reaction, the reactant was poured into 10 mL of water, extracted with 3×10 mL of chloroform, and the chloroform layer was washed with water 4~5 times, then use anhydrous MgSO 4 Dry, filter to obtain the filtrate, remove chloroform to obtain 1.84 g of light yellow liquid N-decyl imidazole, yield 88.3%.
[0052] 1 H-NMR (400 MHz, CDCl 3 ) d H : 7.59 (1H, s, imidazole ring 2-CH), 7.07 (1H, s, imidazole ring 4-CH), 6.91 (1H, s, imidazole ring 5-CH), 3.94 (2H, t, J = 7.2 Hz, -CH linked to the imidazole ring 2 -), 1.78 (2H, m, -CH 2 -), 1.28 (10H, m, -(CH 2 ) 7 -), 0.87 (3H, t, ...
Embodiment 3
[0060] The second step: the synthesis of N-dodecyl imidazole
[0061] Add 0.440 g (11.0 mmol) NaOH, 0.714 g (10.5 mmol) imidazole and 10 mL dimethylsulfoxide (DMSO) into a three-necked flask equipped with a stirrer and a thermometer, and stir at 20°C to 25°C under nitrogen protection until To a transparent solution, 2.49 g (10.0 mmol) of dodecane bromide was added dropwise, and after about 4~6 h of reaction, the reactant was poured into 10 mL of water, extracted with 3×10 mL of chloroform, and then washed with chloroform Layer 4~5 times, then use anhydrous MgSO 4 Dry, filter to obtain the filtrate, remove chloroform to obtain 2.08 g of light yellow liquid N-dodecyl imidazole, yield 88.1%.
[0062] 1 H-NMR (400 MHz, CDCl 3 ) d H : 7.70 (1H, s, 2-CH of imidazole ring), 7.09 (1H, s, 4-CH of imidazole ring), 6.93 (1H, s, 5-CH of imidazole ring), 3.96 (2H, t, J = 7.2 Hz, -CH linked to the imidazole ring 2 -), 1.78 (2H, m, -CH 2 -), 1.24-1.28 (10H, m, -(CH 2 ) 9 -), 0.88 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com