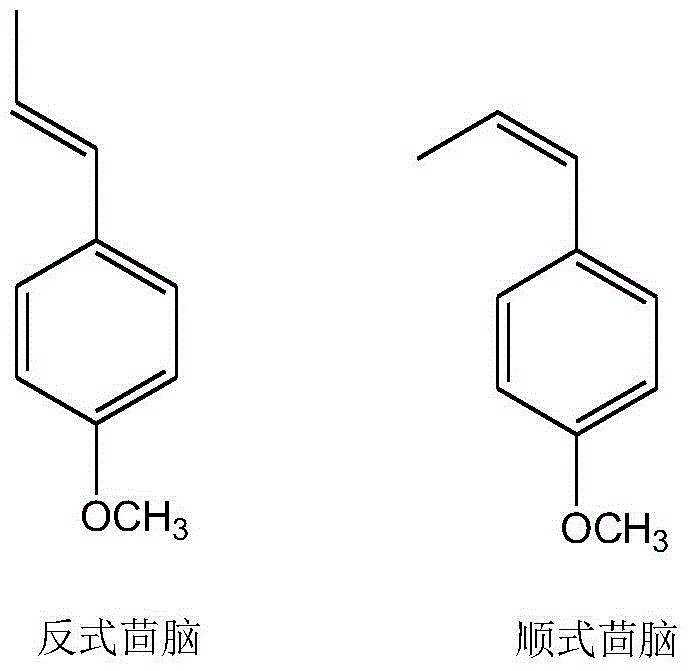
Preparation method of intermediate for synthesizing anise camphor
A kind of technology of anethole and intermediates, applied in the field of production process of organic spices, can solve the problems of high cost, low conversion rate or yield, and conversion rate of only 71%
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0027] According to the present invention, a kind of preparation method of the intermediate of synthetic anethole is provided, and this preparation method comprises following 3 steps:
[0028] Step 1. Add aluminum-nickel alloy in batches to the alkaline aqueous solution, react the aluminum in the alloy completely, pour off the supernatant liquid, add water to wash until neutral, pour off the upper water layer, add solvent and wash until the water content is less than 2% to prepare a Raney nickel catalyst and store it under a solvent cover.
[0029] According to the present invention, at first synthesize Raney nickel catalyst, preferably W-2 type Raney nickel, can adopt existing preparation W-2 type Raney nickel preparation method to prepare, but must control washing water to neutrality during washing, finally use The solvent is washed until the water content is less than 2%, preferably less than 1.5%, more preferably less than 1%, which is particularly important for the subseq...
Embodiment 1
[0053] Embodiment 1: the preparation of Raney nickel catalyst
[0054] Into a 4L four-neck flask, throw 380g of sodium hydroxide dissolved in 1.5L of water, stir, and cool to 10°C under an ice bath. Under stirring, add 300g of aluminum-nickel alloy in batches to the liquid caustic soda in small amounts, the speed of adding should be controlled so that the temperature of the solution does not exceed 25°C (under ice bath). When all the addition was complete, the stirring was stopped, the ice bath was removed, and the reaction solution was allowed to rise to room temperature. When the hydrogen generation rate is slow, it can be heated slowly on a boiling water bath (avoid heating up too fast, in case there are too many bubbles and the reaction solution overflows), until the bubbles slow down again. Then let it stand still, let the nickel powder sink, pour off the supernatant, add water to the original volume, stir the solution to suspend the nickel powder, let it stand again to ...
Embodiment 2
[0055] Example 2: 1-(4-methoxyphenyl)propanol
[0056] In a 1L autoclave, add 300g of p-methoxypropiophenone and 300g of methanol, heat to 60°C, dissolve the p-methoxypropiophenone solid under stirring, then add 30g of Raney nickel prepared in Example 1, and use nitrogen gas After replacing three times, hydrogen gas was introduced, and the temperature was raised slowly. The temperature was controlled at 80°C, and the hydrogen pressure was controlled at 2.0 MPa. After 5 hours of hydrogenation reaction, the reaction did not absorb hydrogen, and the hydrogenation reaction was stopped.
[0057] The temperature was lowered to room temperature, the catalyst was recovered by filtration, and the content of the filtrate was detected by gas chromatography to be 93%. Methanol was recovered by atmospheric distillation, and 275 g of 1-(4-methoxyphenyl)propanol (GC content: 98.5%) was obtained by vacuum distillation at the bottom, with a yield of 90.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com