Applications of ursolic acid derivatives in preparation of drug for preventing and treating tumor metastasis
A technology of tumor metastasis and ursolic acid, applied in anti-tumor drugs, drug combinations, pharmaceutical formulations, etc., can solve the problems of difficult preparation of preparations, low in vivo bioavailability, and no research on normal cytotoxicity and anti-tumor metastasis. selective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
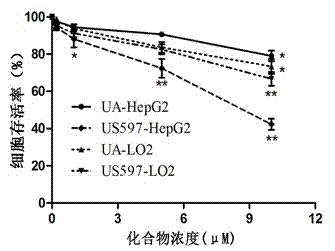
[0031] Example 1 Study on Inhibition of Proliferation of Hepatoma Cell HepG2 by Ursolic Acid and Its Derivatives and LO2 Toxicity to Normal Liver Cells
[0032] After digesting hepatoma cells HepG2 in the logarithmic growth phase and human normal liver cells LO2, the cell density was adjusted to 1×10 5 cells / mL, inoculated in 96-well plate, 100 ??l per well, placed at 37°C, 5% CO 2 Cultivate in the incubator for 24 h; remove the old medium, add the test drug (ursolic acid and its derivatives) and dilute the test drug stock solution with the medium, set different concentrations, 100??l per well, A blank control group was also set up, with 5 replicate wells in each group. After 24 hours of drug action, discard the drug-containing medium, add 100??l of serum-free phenol red-free 1640 medium to each well, and then add 100??l of 0.5 mg / ml MTT solution, continue incubation for 4h, and terminate Culture; carefully discard the supernatant in the wells of the 96-well plate, add 100??...
Embodiment 2
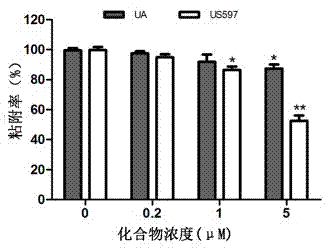
[0034] Example 2 Detection of ursolic acid and its derivatives inhibiting the adhesion of liver cancer cell HepG2 to extracellular matrix
[0035] The fibronectin FN (fibronectin) stored at -20°C was placed in a 37°C water bath until it was completely melted, and the serum-free culture medium was used to prepare the FN working solution with a concentration of 10 μg / ml. Use 100 μL / well FN working solution to completely cover the 96-well plate, leave it at room temperature overnight, and gently suck off the liquid. Take HepG2 cells in the logarithmic growth phase and make a single cell suspension with a cell concentration of 5×10 5 / ml, mixed with different concentrations of drugs (0, 0.2, 1, 5μM), inoculated in FN-coated 96-well plates. 37°C, 5% CO 2 After incubation for 2 h, lightly wash with PBS 3 times, add 10 μl of MTT culture solution after drying, continue to incubate for 4 h, discard the supernatant, add 100 μl DMSO, and detect the OD value at 570 nm with an enzyme-li...
Embodiment 3
[0036] Example 3 Ursolic acid and its derivatives inhibit the adhesion of liver cancer cells HepG2 and HUVEC cells
[0037] Human umbilical vein endothelial cells were isolated from umbilical veins of normal gestational deliveries and prepared according to Jaffe, E. A.; Nachman, R. L.; Becker, C. G.; Minick, C. R. Culture of human endothelial cells derived from umbilicalveins: Identification by morphologic and immunological criteria.Clin. In est. 1973, 52, 2745-2756. The cultivation was carried out. Digest the HUVEC cells in the logarithmic phase and inoculate them in a 24-well plate. When the endothelial cells in the 24-well plate are full of the plate, wash it two or three times with PBS, and then add the endothelial stimulating factor IL-1β at a concentration of 1 ng / L medium at 37 °C, 5% CO 2 Incubate for 4 h under conditions. After 4 hours, take out the well plate, wash it two or three times with PBS, take HepG2 cells in the logarithmic growth phase, and make 4×10 cell...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com