Reducibly degradable amphiphilic block copolymer and preparation and application of amphiphilic block copolymer used as drug carrier
An amphiphilic block and copolymer technology, applied in the fields of polymer chemistry and biomedicine, can solve problems such as toxic and side effects, reducing the effect of drugs on the target site, and increasing the drug resistance of tumor cells.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] (1) Preparation of disulfide bond monomer: Weigh 1.54 g (10 mmol) of 2,2-dithioethanol, dissolve it in a round-bottomed flask with 10 mL of THF, and place it in an ice-water bath. Add 3 mL of triethylamine (20 mmol) dropwise to the round bottom flask and stir for 20 min. Dissolve 0.5 mL of freshly prepared methacryloyl chloride (4.8 mmol) in 5 mL of THF and slowly add it dropwise to the above reaction flask. After the dropwise addition was completed, it was stirred in an ice-water bath for 2 h, and left at room temperature overnight. After removing the solid product by filtration. After the THF was removed by vacuum rotary evaporation, 50 mL of dichloride was added to dissolve the solid product, and the filtrate was washed with dilute hydrochloric acid solution (mass concentration 2%), sodium bicarbonate solution (mass concentration 2%), high-purity water, and dried over anhydrous magnesium sulfate. Layer, 0.58 g of disulfide bond monomer compound was obtained with a ...
Embodiment 2
[0047] (1) Preparation of disulfide bond monomer: same as Example 1;
[0048] (2) Preparation of grafted Rhodamine B disulfide bond monomer: same as Example 1;
[0049] (3) Preparation of intermediate interpolymer: same as Example 1;
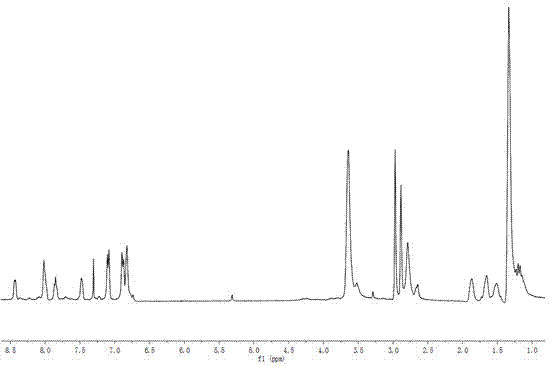
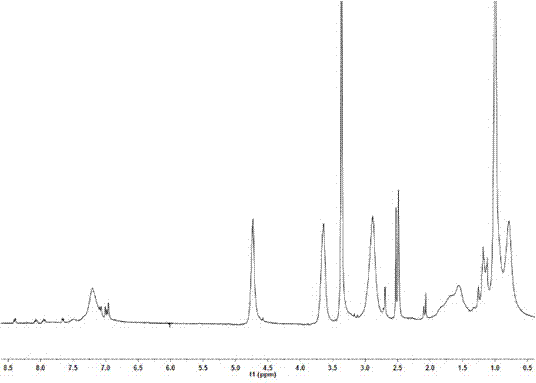
[0050] (4) Preparation of the target copolymer: Weigh 0.050 g of the intermediate copolymer, 0.329 g (2.30 mmol, 96.6%) N-(2-hydroxypropyl)methacrylamide (HPMA), put it in a Shleck bottle, and use Dissolve 2 mL of DMSO; add 0.0189 g (5%, wt) azobisisobutyronitrile (AIBN), vacuumize and inflate with nitrogen for 3 to 5 times, seal and keep the temperature at about 55°C for 24 h. Precipitate with petroleum ether, centrifuge with an ultrafiltration concentration centrifuge tube with a molecular weight of 3000, remove small molecules, and obtain 0.130 g of polymer with a yield of about 34.3%. Mn=1.45×10 4 , Mw / Mn=1.37. 1 H NMR (400 MHz, DMSO, δ, ppm): 8.41- 6.84 (Ar), 3.65 (CH 3 C H (OH)CH2 NH- of HPMA and -N(C H 2 CH 3 ) 2 ), 2.89 (CH 3 ...
Embodiment 3
[0053] (1) Preparation of disulfide bond monomer: same as Example 1;
[0054] (2) Preparation of grafted Rhodamine B disulfide bond monomer: same as Example 1;
[0055] (3) Preparation of intermediate interpolymer: same as Example 1;
[0056] (4) Preparation of the target copolymer: Weigh 0.025 g of the intermediate copolymer, 0.128 g (0.89 mmol, 95.7%) N-(2-hydroxypropyl)methacrylamide (HPMA), put it in a Shleck bottle, and use Dissolve 2 mL of DMSO; add 0.0122 g (8%, wt) of azobisisobutyronitrile (AIBN), vacuumize and inflate with nitrogen for 3 to 5 times, keep the temperature at about 55°C after sealing, and react for 24 h. Precipitate with petroleum ether, centrifuge with an ultrafiltration concentration centrifuge tube with a molecular weight of 3000, remove small molecules, and obtain 65 mg of polymer with a yield of about 42.4%. Mn=1.30×10 4 , Mw / Mn=1.25. 1 H NMR (400 MHz, DMSO, δ, ppm): 8.46- 6.85 (Ar), 3.62 (CH 3 C H (OH)CH 2 NH- of HPMA and -N(C H 2 CH 3 )...
PUM
| Property | Measurement | Unit |
|---|---|---|
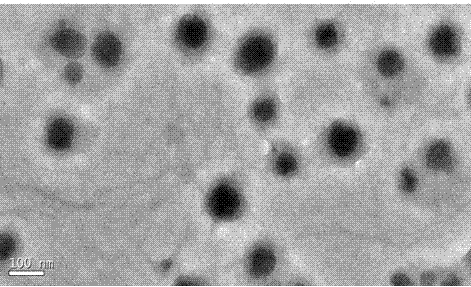
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com