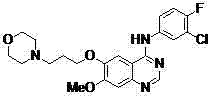
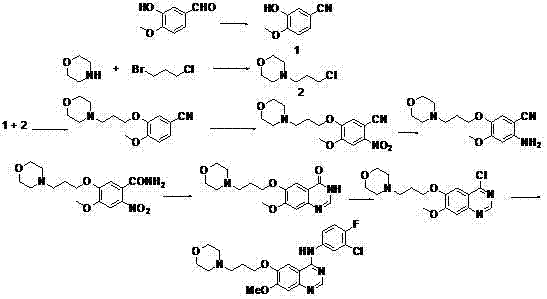
New method for performing microwave synthesis on gefitinib and derivative thereof
A technology for gefitinib and microwave synthesis, which is applied in the new field of microwave synthesis of gefitinib and its derivatives, can solve the problems of low total yield and many reaction steps, and achieve simple operation, reduced product cost, and high production efficiency. high rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The first step: the preparation of 6-methoxy-7-hydroxyquinazolin-4-one
[0035]
[0036] Weigh 1 mmol of 2-iodo(bromo)-4-methoxy-5-hydroxybenzonitrile, 1.5 mmol of formamidine hydrochloride in 10 mL of water phase, add 0.1 mmol of CuI catalyst, base K 2 CO 3 3 mmol, 80 ° C microwave heating reflux 0.5 h, microwave power 150 W. After the reaction was completed, it was cooled to room temperature and filtered to obtain solid powder.
[0037] The second step: the preparation of 7-methoxy-6-[3-(4-morpholinyl)propoxy]quinazolin-4-one
[0038]
[0039] Weigh 1 mmol of 7-methoxy-7-hydroxyquinazolin-4-one and 1.2 mmol of 4-(3-bromopropyl)morpholine, add 0.1 mmol of CuI catalyst to 10 mL of water phase, base K 2 CO 3 3 mmol, 90 ° C microwave heating reflux 0.5 h, microwave power 150 W. After the reaction was completed, it was cooled to room temperature and filtered to obtain solid powder.
[0040] The third step: the preparation of 4-chloro-7-methoxy-6-[3-(4-morphol...
Embodiment 2
[0047] Preparation of 4-(3-chloro-4-fluoroanilino)-7-methyl-6-(3-morpholinopropoxy)quinazoline
[0048]
[0049] Weigh 1 mmol of 4-chloro-7-methyl-6-[3-(4-morpholinyl)propoxy]quinazoline and 1.2 mmol of 3-fluoro-4-chloroaniline in 10 mL of aqueous phase, add CuI Catalyst 0.1mmol, base K 2 CO 3 2 mmol, 100 ° C microwave heating reflux 0.5 h, microwave power 150 W. After the reaction solution was cooled to room temperature, excess reaction raw materials were removed by filtration, and the filtrate was distilled under reduced pressure to precipitate a solid, which was recrystallized from ethyl acetate to obtain a white solid, which was a gefitinib derivative.
Embodiment 3
[0051]Preparation of 4-chloro-7-methoxy-6-[3-(4-morpholinyl)propoxy]quinazoline
[0052] 1mmol of 7-methoxy-6-[3-(4-morpholino)propoxy]quinazolin-4-one with 2mmol of chlorination reagent phosphorus trichloride and 2mmol of base Na 2 CO 3 Microwave reaction in the presence of intermediate 4-chloro-7-methoxy-6-[3-(4-morpholinyl)propoxy]quinazoline. The reaction temperature was 80 °C, and the microwave power was 150 W. After the reaction was completed, it was cooled to room temperature and filtered to obtain solid powder.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com