Anticancer nano medicament loading arsenical and preparation method thereof
A nano-drug and nano-sphere technology, which is applied in the direction of anti-tumor drugs, drug combinations, and pharmaceutical formulations, can solve the problems of unfavorable drug efficacy and fast metabolism, and achieve the effects of easy scale-up preparation, simple process steps, and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1: Preparation of hollow silica nanomaterials with a radius of 30 nm
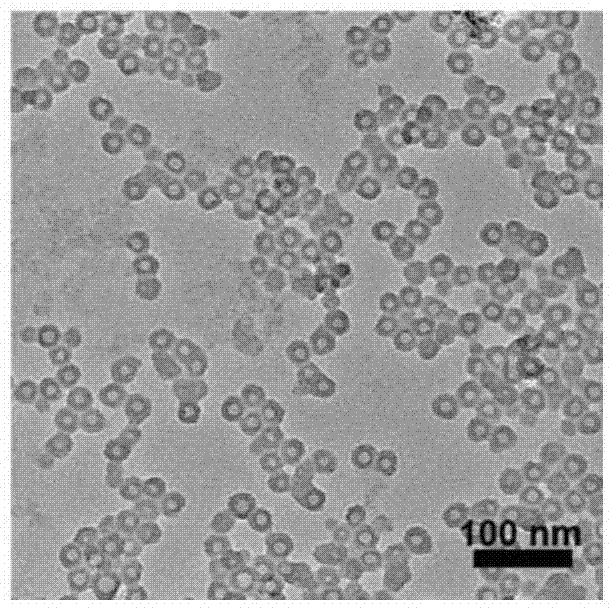
[0033]In a 50 mL round bottom flask, 60 mg of iron oxide nanoparticles with uniform particle size distribution were added and dissolved in 20 mL of cyclohexane, while 900 μL of Co-520, 200 μL of tetraethoxysilane and 400 μL of ammonia were added. The mixed solution was stirred (rotating speed: 800rpm), mixed evenly, and kept at the rotating speed for 16h. Then, 10 μL of 3-aminopropyltriethoxysilane was added to the reaction liquid and reacted for 2 h. After the reaction was completed, about 1 mL of absolute ethanol was added dropwise to the reaction liquid and centrifuged at 5000 rpm for 2 min. The resulting precipitate was redissolved in 10 mL of ultrapure aqueous solution to obtain hollow silica nanoparticles with a radius of 30 nm (see figure 2 ). figure 1 A schematic diagram of the anticancer nanomedicine loaded with arsenic agent according to the present invention is given.
Embodiment 2
[0034] Example 2: Ni,AsSiO 2 Preparation of anticancer nanomedicine
[0035] Add 2 mL of hollow silica nanoparticle solution with a concentration of 10 mg / mL and 3 mL of 600 mM nickel acetate solution in a 10 mL round bottom flask. The mixed solution was placed in a water bath at 50°C and an ice-water bath at 0°C in sequence, and circulated 10 times. After 10 rounds of cycles were completed, the excess nickel acetate solution was discarded by centrifugation (14000 rpm), and 3 mL of 150 mM ATO aqueous solution (pH 8) was added thereto. The mixture was placed in a 50°C water bath for 6h. After the reaction is complete, centrifuge at 14,000rpm for 30min, add 2mL of ultrapure water to dissolve the precipitate, and obtain Ni, AsSiO 2 Anticancer nanomedicines (see image 3 and 4 ).
Embodiment 3
[0036] Example 3: Mn, AsSiO 2 Preparation of anticancer nanomedicine
[0037] Add 2 mL of hollow silica nanoparticle solution with a concentration of 10 mg / mL and 3 mL of 600 mM manganese chloride solution in a 10 mL round bottom flask. The mixed solution was placed in a water bath at 50°C and an ice-water bath at 0°C in sequence, and circulated 10 times. After 10 rounds of cycles were completed, the excess manganese chloride solution was discarded by centrifugation (14000 rpm), and 3 mL of 150 mM ATO aqueous solution (pH 8) was added thereto. The mixture was placed in a 50°C water bath for 6h. After the reaction is complete, centrifuge at 14,000rpm for 30min, add 2mL of ultrapure water to dissolve the precipitate, and obtain Mn, AsSiO 2 Anticancer nanomedicines (see Figure 5 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com