Steroid ester preparation method
A technology of esterification and steroids, which is applied in the field of preparation of steroidal esterification, can solve problems such as poor quality, many impurities, and low product yield, and achieve the effects of simple operation, improved yield and quality, and improved purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
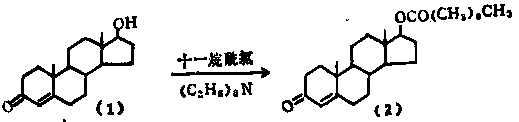
[0030] The preparation method of the steroidal esterification product of the present invention uses the alcoholic hydroxy steroidal compound (I) as the starting material, undergoes the catalysis of a catalyst, uses a dehydrating agent, and undergoes an esterification reaction with the alkanoic acid compound (II) to obtain the steroidal esterification compound (I) Body ester compound (III), its operational route is as follows:
[0031]
[0032] Wherein, R1 in the alcoholic hydroxysteroid compound (I) contains alcoholic hydroxysteroids such as hydrogen, alkanes, enynes, and ketones;
[0033] R2 in the alkanoic acid compound (II) includes C2-C18 alkanoic acids such as acetic acid, pentanoic acid, hexanoic acid, heptanoic acid, octanoic acid, undecanoic acid, and tridecanoic acid.
[0034] Specifically include the following steps:
[0035] In the first step, the alcohol hydroxy steroid compound (I) is put in, the reaction solvent is added, stirred, and the alkanoic acid compou...
Embodiment 1
[0042] Preparation of testosterone undecanoate:
[0043] Put 10g of steroidal alcohol testosterone into the reaction flask, add 200ml of reaction solvent chloroform, stir, add 20g of undecanoic acid, add 5g of catalyst DMAP and dehydrating agent N,N-diisopropylcarbodiimide (DIC ) 20g combination, reacted at 5-35°C for 5 hours, added water to terminate, put in 20g of alumina as an acid removal substance and stirred for 30 minutes; filtered, extracted, separated, combined and concentrated the organic phases to obtain the compound testosterone undecanoate; yield 155%, HPLC≧98%.
Embodiment 2
[0045] Preparation of norethindrone enanthate:
[0046] Put 10 g of steroid norethindrone into the reaction flask, add 200 ml of reaction solvent chloroform, stir, add 20 g of heptanoic acid, add 5 g of catalyst DMAP and dehydrating agent 1-ethyl-(3-dimethylaminopropyl) carbon Diimine hydrochloride (EDCI) 20g combination, react at 5-35°C for 5 hours, add water to terminate, add 20g calcium oxide as an acid removal substance and stir for 30 minutes; filter, extract, separate layers, combine organic phases and concentrate to obtain compound 11 Testosterone acid; yield 120%, HPLC≧98%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com