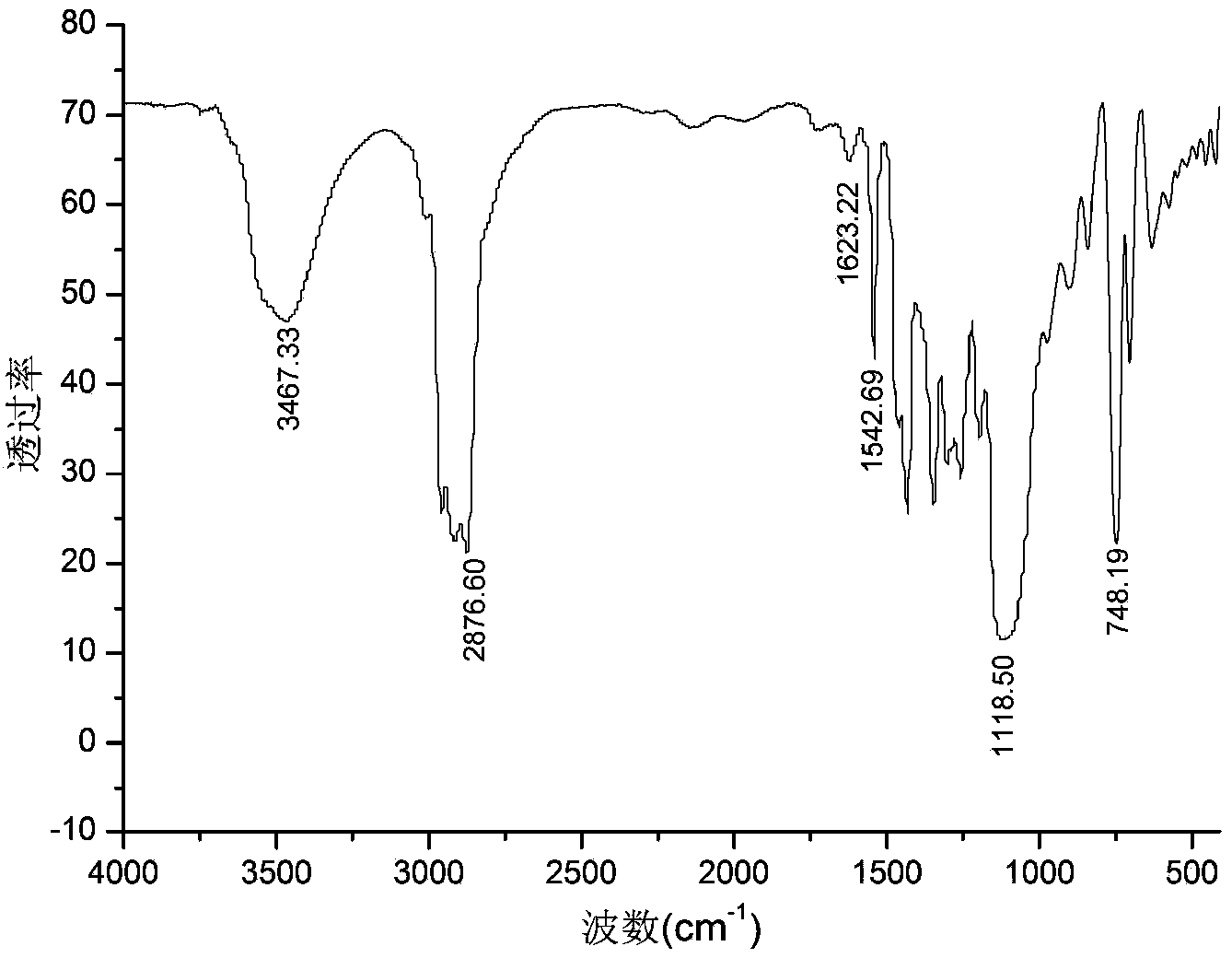
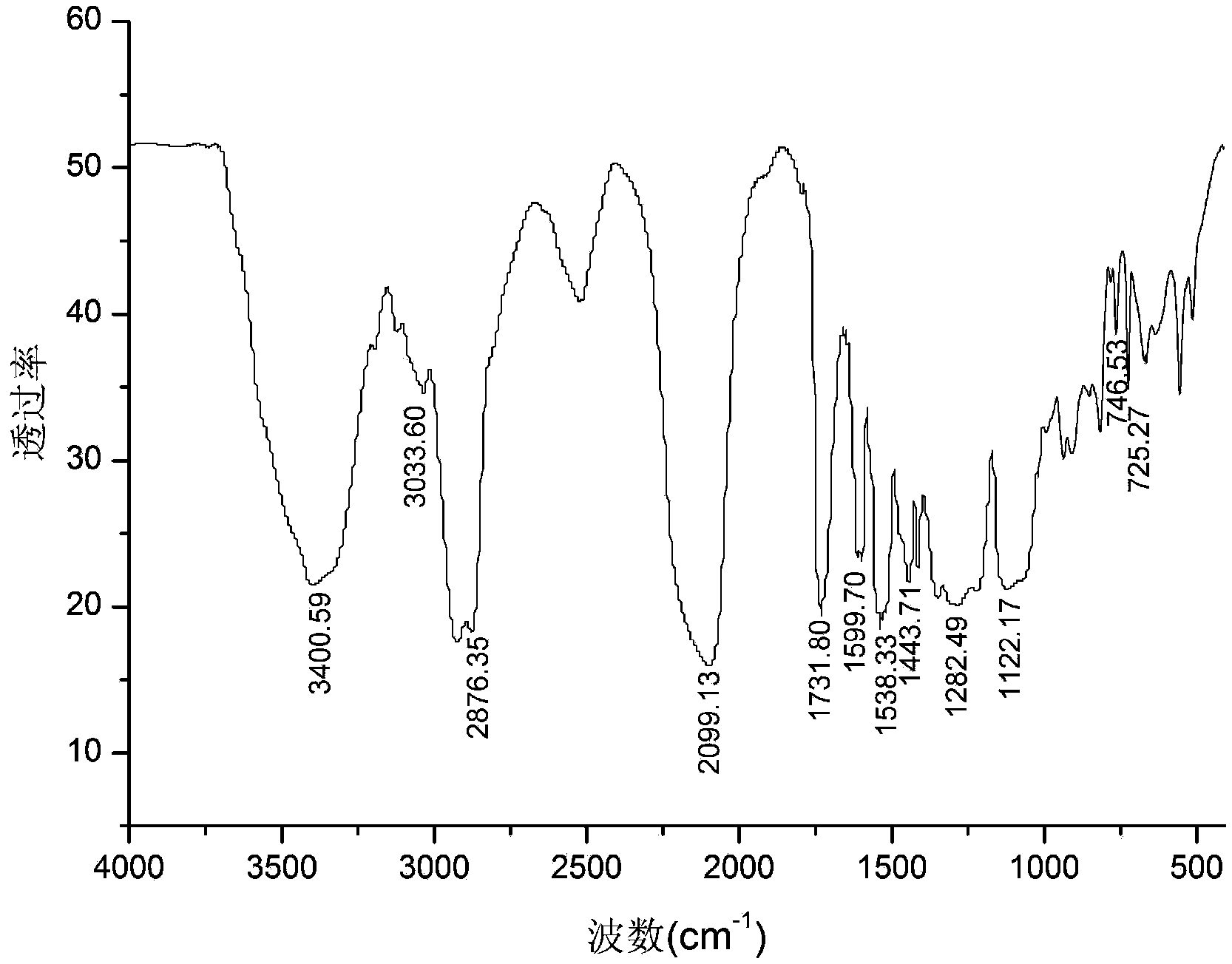
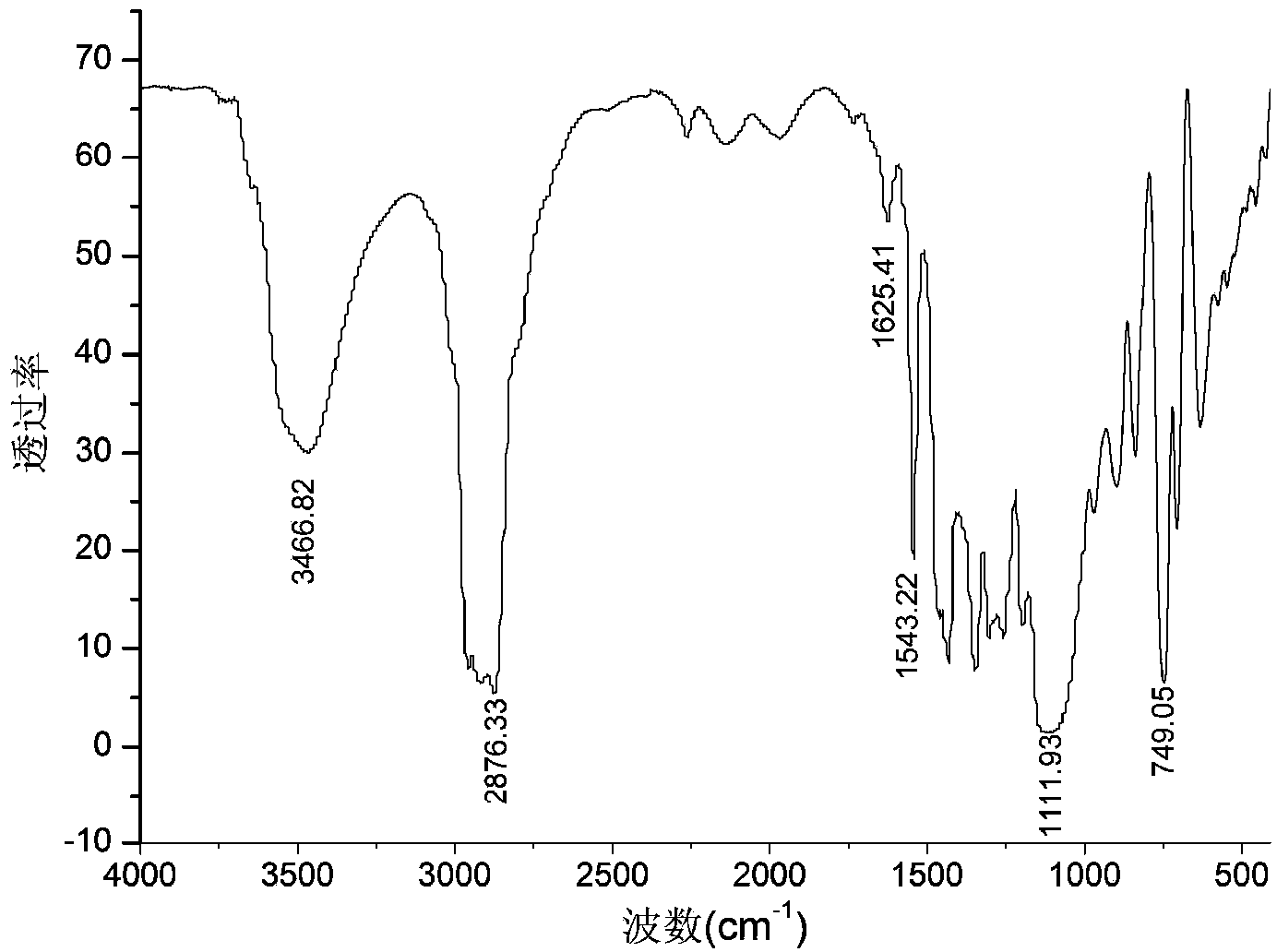
Method for preparing double-energy-containing-group polyurethane elastomer
A polyurethane elastomer and dual-energy technology, which is applied in the field of preparation of dual-energy-containing polyurethane elastomers, can solve problems such as large structural differences, poor trinitrotoluene compatibility, and reduced energy of thermoplastic elastomers, and achieve enhanced Intermolecular interaction, enhancing the effect of interaction between benzene rings
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Add epichlorohydrin dropwise to 2,6-dinitro-p-dibenzyl alcohol and tin tetrachloride methylene chloride solution at a constant temperature of 30°C, wherein 2,6-dinitro-p-dibenzyl alcohol and epichlorohydrin The molar ratio of 1:100, adding 3.0% tin tetrachloride mass fraction. The metered epichlorohydrin was added dropwise within 2 hours, and reacted for 3 hours under constant temperature stirring to obtain a polyepichlorohydrin solution.
[0025] The polyepichlorohydrin solution was added to water at 60°C for washing, the upper aqueous solution was discarded, and the organic matter in the lower layer was distilled under reduced pressure to obtain polyepichlorohydrin. The obtained polyepichlorohydrin has a number average molecular weight of 8670 and a weight average molecular weight of 14500 as measured by gel chromatography.
[0026] Under the protection of nitrogen, the temperature is 110 ° C, sodium azide and polyepichlorohydrin in N, N-dimethylformamide solvent to ...
Embodiment 2
[0031] Add epichlorohydrin dropwise to 2,6-dinitro-p-dibenzyl alcohol and tin tetrachloride methylene chloride solution at a constant temperature of 30°C, wherein 2,6-dinitro-p-dibenzyl alcohol and epichlorohydrin The molar ratio of 1:50, adding 3.0% tin tetrachloride mass fraction. The metered epichlorohydrin was added dropwise within 2 hours, and reacted for 3 hours under constant temperature stirring to obtain a polyepichlorohydrin solution.
[0032] The polyepichlorohydrin solution was added to 60°C water for washing, the upper aqueous solution was discarded, and the organic matter in the lower layer was distilled under reduced pressure to obtain polyepichlorohydrin. The number average molecular weight of the obtained polyepichlorohydrin measured by gel chromatography is 4210, and the weight average molecular weight is 6100.
[0033] Under the protection of nitrogen, the temperature is 120 ° C, sodium azide and polyepichlorohydrin in N, N-dimethylformamide solvent to synt...
Embodiment 3
[0038] Add epichlorohydrin dropwise to 2,6-dinitro-p-dibenzyl alcohol and tin tetrachloride methylene chloride solution at a constant temperature of 30°C, wherein 2,6-dinitro-p-dibenzyl alcohol and epichlorohydrin The molar ratio of 1:10, adding 3.0% tin tetrachloride mass fraction. The metered epichlorohydrin was added dropwise within 2 hours, and reacted for 3 hours under constant temperature stirring to obtain a polyepichlorohydrin solution.
[0039] The polyepichlorohydrin solution was added to water at 60°C for washing, the upper aqueous solution was discarded, and the organic matter in the lower layer was distilled under reduced pressure to obtain polyepichlorohydrin. The number average molecular weight of the obtained polyepichlorohydrin measured by gel chromatography is 3480, and the weight average molecular weight is 6030.
[0040] Under the protection of nitrogen, the temperature is 100 ° C, sodium azide and polyepichlorohydrin in N, N-dimethylformamide solvent to s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com