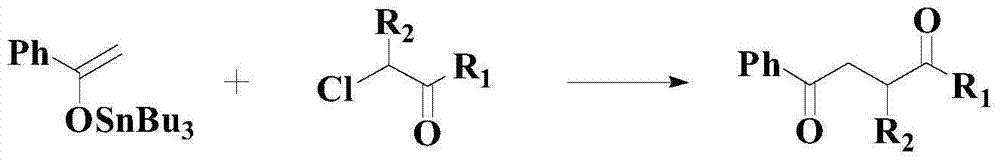
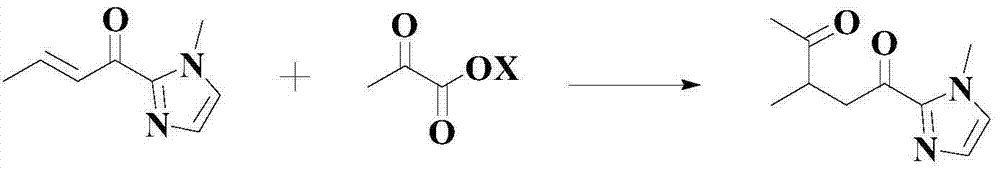
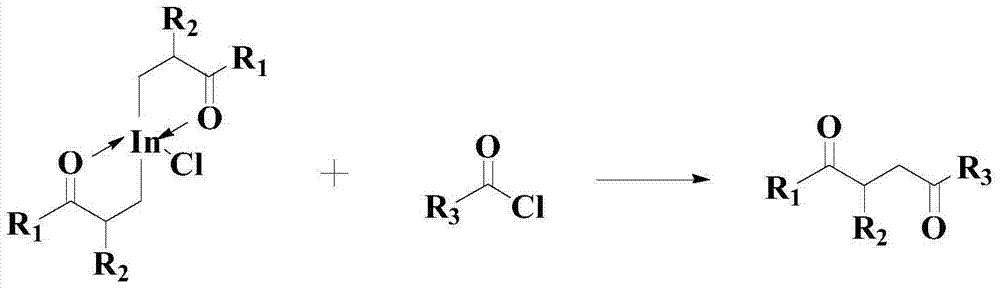
Improved catalyzed synthesis method for 1,4-dicarbonyl compound
A dicarbonyl compound and synthesis method technology, applied in the field of chemical intermediate synthesis, can solve the problems of low reaction yield, small substrate application range, cumbersome post-treatment, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038]
[0039] Weigh Cu(acac) according to the molar ratio 1:0.2:0.6 2 , phthalocyanine and 4,4'-dimethyl-2,2'-bipyridine, after they were ground and mixed uniformly, the composite catalyst used in this embodiment was obtained.
[0040] Add 10mmol of compound of formula (I) and 30mmol of compound of formula (II) into the reactor, then sequentially add 50ml of triethylamine, 0.4mmol of composite catalyst, 50mmol of TBHP, seal and incubate at 55°C for 12 hours. After the reaction was completed, the reaction was quenched by adding saturated aqueous sodium thiosulfate solution, the resulting mixture was extracted with ethyl acetate, the organic phase was dried with anhydrous magnesium sulfate, and then the extraction solvent was removed by evaporation, and the residue was purified by column to obtain the compound of formula (III), The eluent used for the column purification is a mixture of petroleum ether / ethyl acetate, wherein the volume ratio of petroleum ether to ethyl acet...
Embodiment 2
[0043]
[0044] Weigh Cu(acac) according to the molar ratio 1:0.1:0.8 2 , phthalocyanine and 4,4'-dimethyl-2,2'-bipyridine, after they were ground and mixed uniformly, the composite catalyst used in this embodiment was obtained.
[0045] Add 10mmol of the compound of formula (I) and 20mmol of the compound of formula (II) into the reactor, then sequentially add 40ml of triethylamine, 0.3mmol of composite catalyst, and 40mmol of TBHP, and seal and incubate at 60°C for 10 hours. After the reaction was completed, the reaction was quenched by adding saturated aqueous sodium thiosulfate solution, the resulting mixture was extracted with ethyl acetate, the organic phase was dried with anhydrous magnesium sulfate, and then the extraction solvent was removed by evaporation, and the residue was purified by column to obtain the compound of formula (III), The eluent used for the column purification is a mixture of petroleum ether / ethyl acetate, wherein the volume ratio of petroleum eth...
Embodiment 3
[0048]
[0049] Weigh Cu(acac) according to the molar ratio 1:0.3:0.5 2 , phthalocyanine and 4,4'-dimethyl-2,2'-bipyridine, after they were ground and mixed uniformly, the composite catalyst used in this embodiment was obtained.
[0050] Add 10mmol of compound of formula (I) and 10mmol of compound of formula (II) into the reactor, then sequentially add 45ml of triethylamine, 0.1mmol of composite catalyst, 45mmol of TBHP, seal and incubate at 50°C for 15 hours. After the reaction was completed, the reaction was quenched by adding saturated aqueous sodium thiosulfate solution, the resulting mixture was extracted with ethyl acetate, the organic phase was dried with anhydrous magnesium sulfate, and then the extraction solvent was removed by evaporation, and the residue was purified by column to obtain the compound of formula (III), The eluent used for the column purification is a mixture of petroleum ether / ethyl acetate, wherein the volume ratio of petroleum ether to ethyl acet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com