Method for producing 5- hydroxymethyl-2- furfural or alkyl ether derivatives thereof using an ion exchange resin in the presence of an organic solvent
一种交换树脂、有机溶剂的技术,应用在生产基于呋喃的化合物领域,能够解决增加生产和回收过程成本、不理想等问题,达到有利于分离和纯化、转化率高的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-51
[0094] Examples 1-51 (Step 1)
[0095] In each tubular reactor, put 100mg of glucose and 50-300mg of sodium bicarbonate (NaHCO 3 ) or sodium aluminate (NaAlO 4 ) anion exchange resin (Amberlite IRA-400, Amberlite IRA-900, Amberlite IRA-743, Amberlyst A-26) washed with saturated solution (weight ratio of anion exchange resin / glucose (AER / Glu)=0.5-3).
[0096] In each reactor, 3 ml of an organic solvent (DMSO, DMF, ethanol, dioxane, isopropanol) was placed, and the resulting mixture was stirred at 80-100° C. for a predetermined period of time. After the reaction was terminated, each reactor was cooled to room temperature, diluted with HPLC (high performance liquid chromatography) grade distilled water, and analyzed by HPLC to determine the conversion yield of fructose. The samples were separated by HPLC (Agilent 1200 series) with an ion exclusion column (Bio-Rad Aminex HPX-87H300×7.8 mm), and measured by RID detector to determine the conversion yield.
[0097] The anion excha...
experiment Embodiment 1
[0103] Experimental Example 1: Comparison of Fructose Productivity with Changes in Anion Exchange Resin Washing Solution
[0104] In Examples 1-8, after the reaction was terminated, each reactor was cooled to room temperature, diluted with HPLC grade distilled water, and analyzed by HPLC to determine the yield. The sample was separated by HPLC (Agilent 1200 series) with an ion exclusion column (Bio-Rad Aminex HPX-87H300×7.8mm), and was determined using a RID detector to determine the yield.
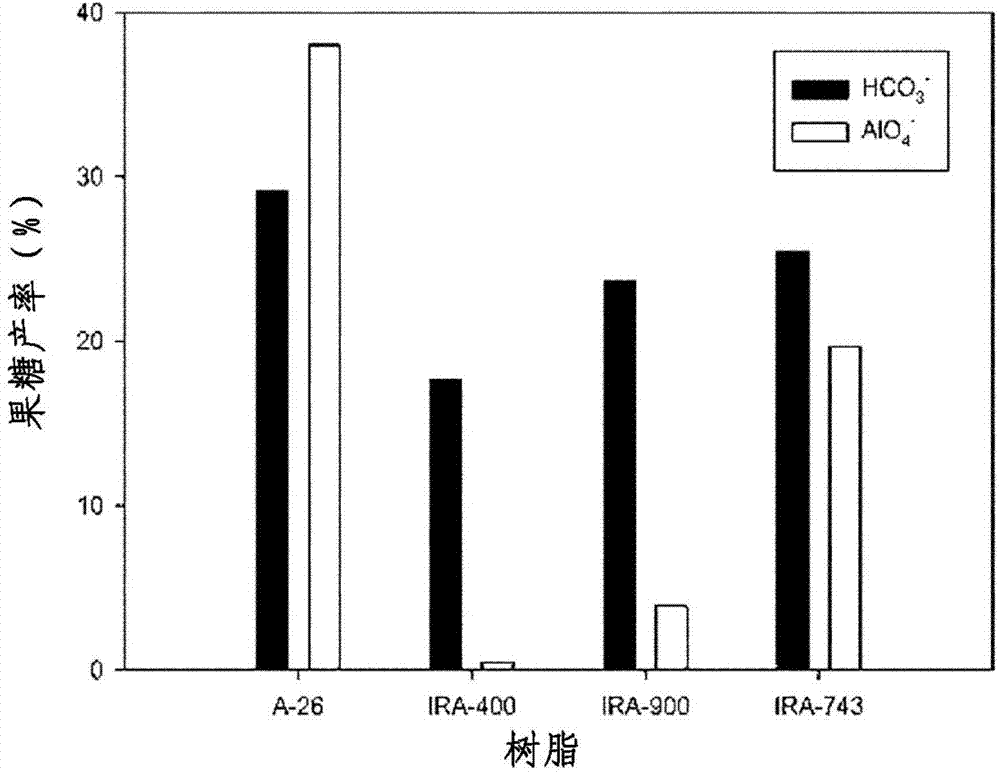
[0105] In embodiment 1-8, fructose productive rate is compared with the change situation comparison result of anion exchange resin washing solution as follows figure 1 shown.
[0106] Such as figure 1 It was shown that anion exchange resin (regardless of its type) washed with sodium bicarbonate can produce fructose in 20%-30% yield. On the other hand, in Example 2 using gel-type Amberlite A-26 washed with sodium aluminate, the yield of fructose was as high as 38%, but in Example 2 usin...
experiment Embodiment 2
[0107] Experimental Example 2: Comparison of Fructose Yield with the Weight Ratio of Anion Exchange Resin / Glucose (AER / Glu)
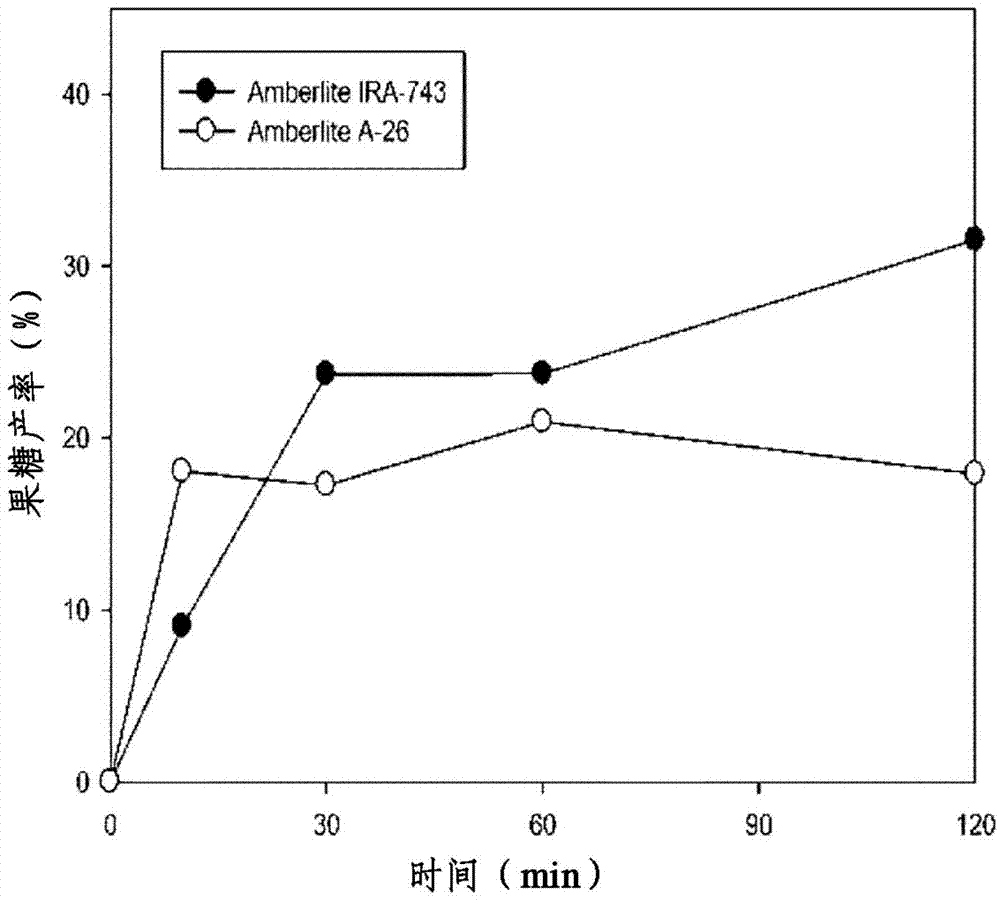
[0108] Reactions in Examples 9-12 (10 minute reaction, Amberlite A-26), Examples 13-16 (30 minute reaction, Amberlite A-26) and Examples 33-36 (10 minute reaction, Amberlite IRA-743) A sample was then taken, diluted with HPLC grade distilled water, and analyzed by HPLC to determine the yield.
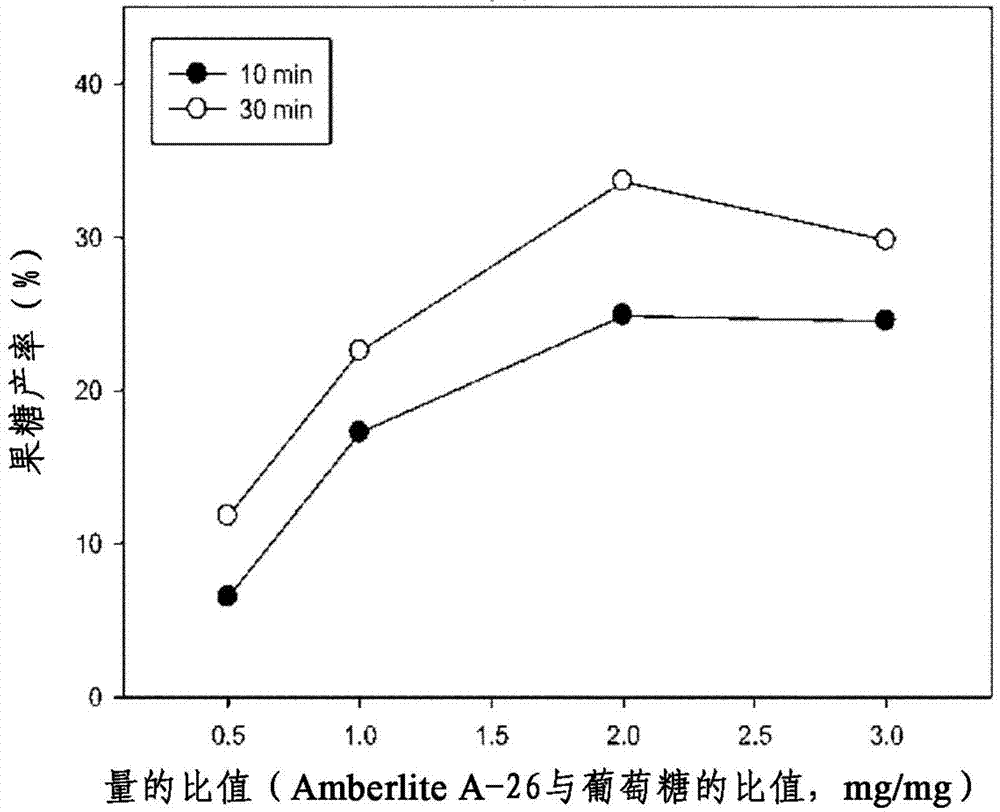
[0109] As a result of the analysis, when the weight ratio of anion exchange resin to glucose was 2 (AER / Glu=2), fructose in Example 11 (25%), Example 15 (34%) and Example 35 (18%) Yields are very high.
[0110] In Examples 9-16, the fructose yield varies with the weight ratio of anion exchange resin / glucose as figure 2 shown. Such as figure 2 As shown, the maximum conversion was at the above weight ratio of 2, and the reaction was continued for 30 minutes.
PUM
| Property | Measurement | Unit |
|---|---|---|
| conversion efficiency | aaaaa | aaaaa |
| conversion efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com