Method using visible light catalyzed cross-coupling reaction to prepare coupling product and release hydrogen
A cross-coupling, visible light technology, applied in the field of cross-coupling hydrogen release, visible light-catalyzed cross-coupling hydrogen release of tertiary amines and nucleophiles, can solve the problems of insufficient driving force, difficult to achieve, etc., to achieve the scope of application Wide, mild reaction conditions, efficient cross-coupling effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
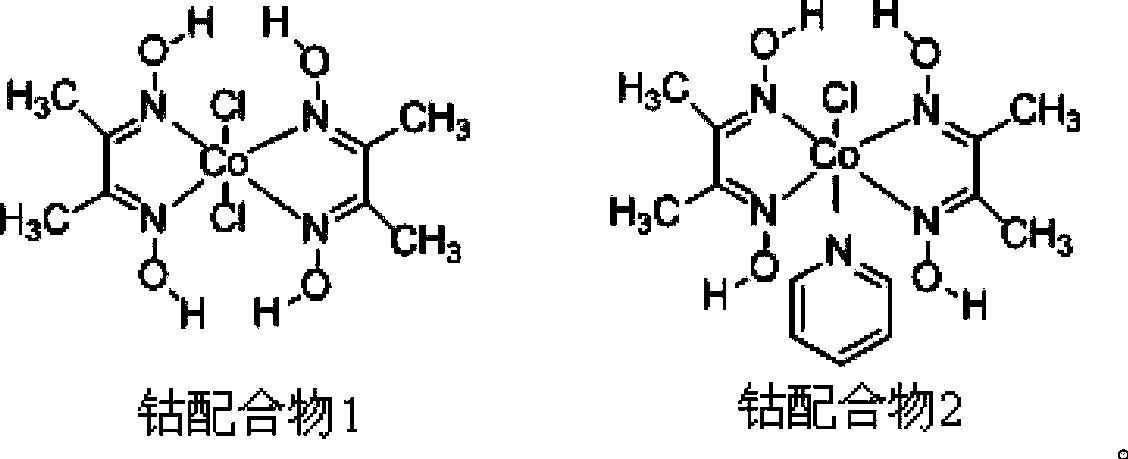
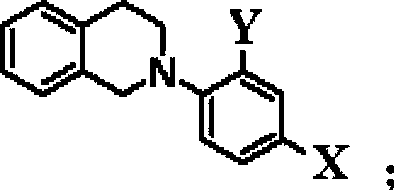
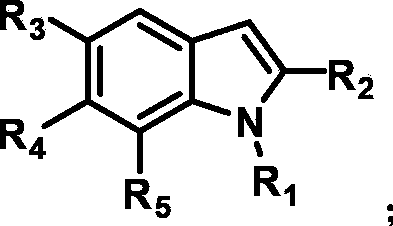
[0032] With cobalt complex 1 as catalyst, 0.36 mg of the catalyst was added to 2 mL of water; with eosinY as photosensitizer, 0.7 mg of the photosensitizer was added to 2 mL of water; the catalyst solution and the photosensitizer solution were mixed to obtain 4 mL of a mixed aqueous solution. Take 0.1mmol tertiary amine-1 (X, Y are H) and 0.2mmol indole-1 (R 1 ﹑ R 2 ﹑ R 3 ﹑ R 4 ﹑ R 5 H) was dissolved in 1 mL of acetonitrile to obtain an acetonitrile solution. Further, 4 mL of the mixed aqueous solution was added to the acetonitrile solution to obtain 5 mL of the mixed solution. Wherein the concentration of photosensitizer is 2.0×10 -4 M, the catalyst concentration is 2.0×10 -4 M. Nitrogen deoxygenated for 30 minutes, sealed with wax, injected with 600 μL of internal standard methane, and then irradiated with 525nm±10nm Green LEDs at 40°C for 12 hours, using gas chromatography (TCD as the detector, Shanghai Technology Scientific Instrument Co., Ltd., Technology 7890II T...
Embodiment 2
[0034] With embodiment 1, difference is: add 1.44mg photosensitizer, the concentration of photosensitizer is 4.0 * 10 -4 M. Approximately 315 μL of hydrogen gas was produced. The product is 1-(1H-indol-3-yl)-2-phenyl-1,2,3,4-tetrahydroisoquinoline. The conversion of the starting material was 15%, the yield of the coupling product was 11%, and the amount of hydrogen evolved was 14% relative to the theoretical value.
Embodiment 3
[0036] With embodiment 1, difference is: add 2.04mg photosensitizer, the concentration of photosensitizer is 6.0×10 -4 M. Approximately 427 μL of hydrogen gas was produced. The product is 1-(1H-indol-3-yl)-2-phenyl-1,2,3,4-tetrahydroisoquinoline. The conversion of the starting material was 21%, the yield of the coupling product was 13%, and the amount of hydrogen evolved was 19% relative to the theoretical value.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com