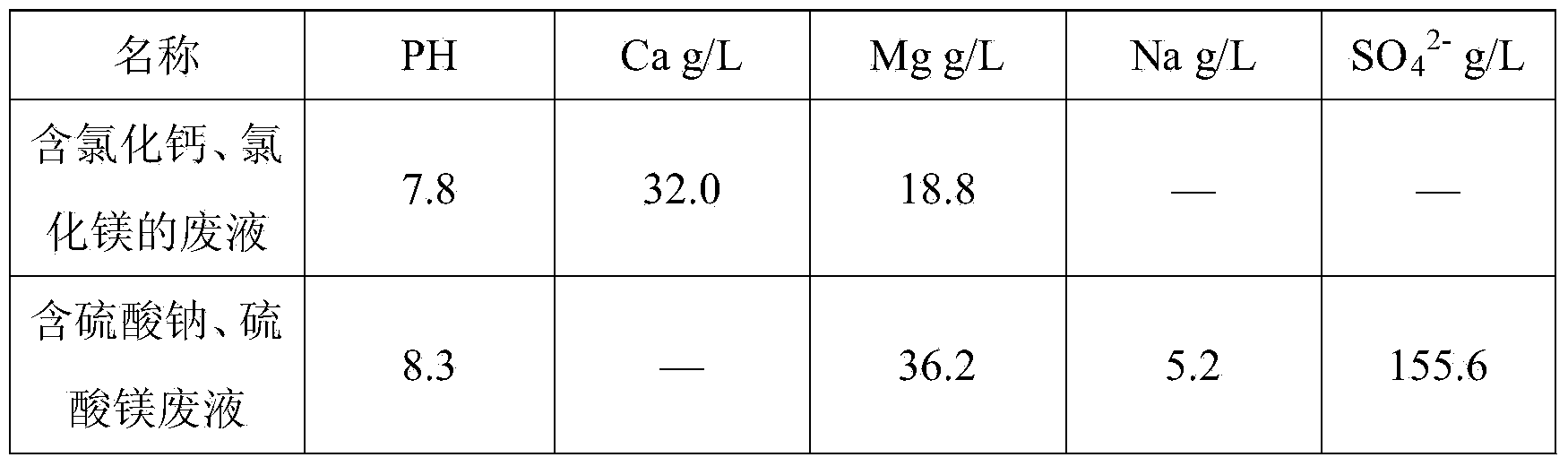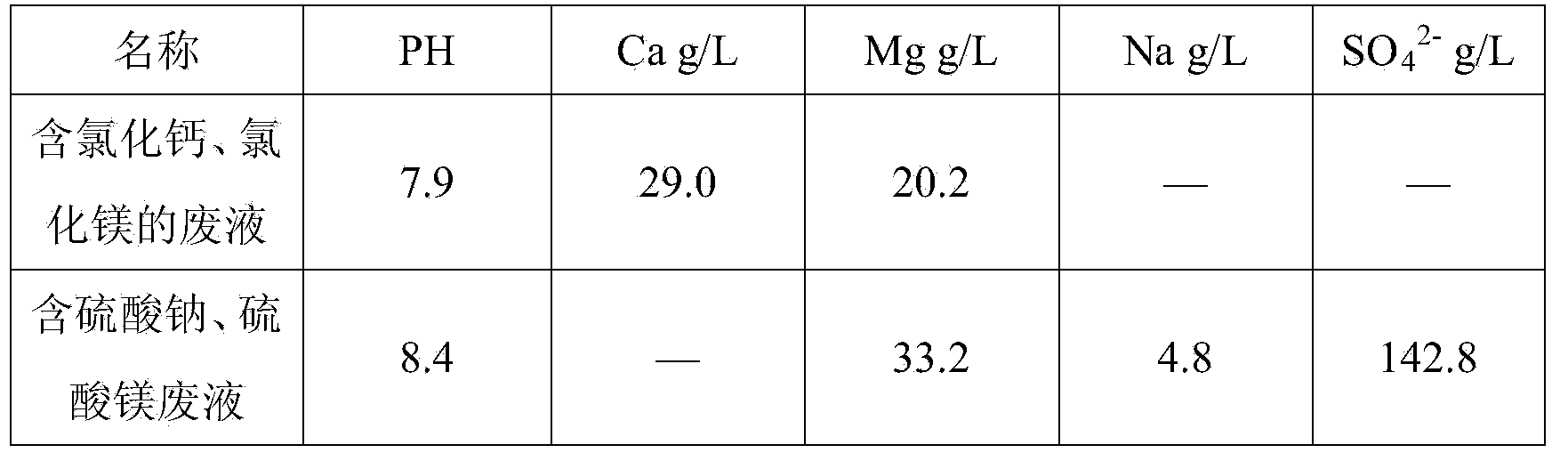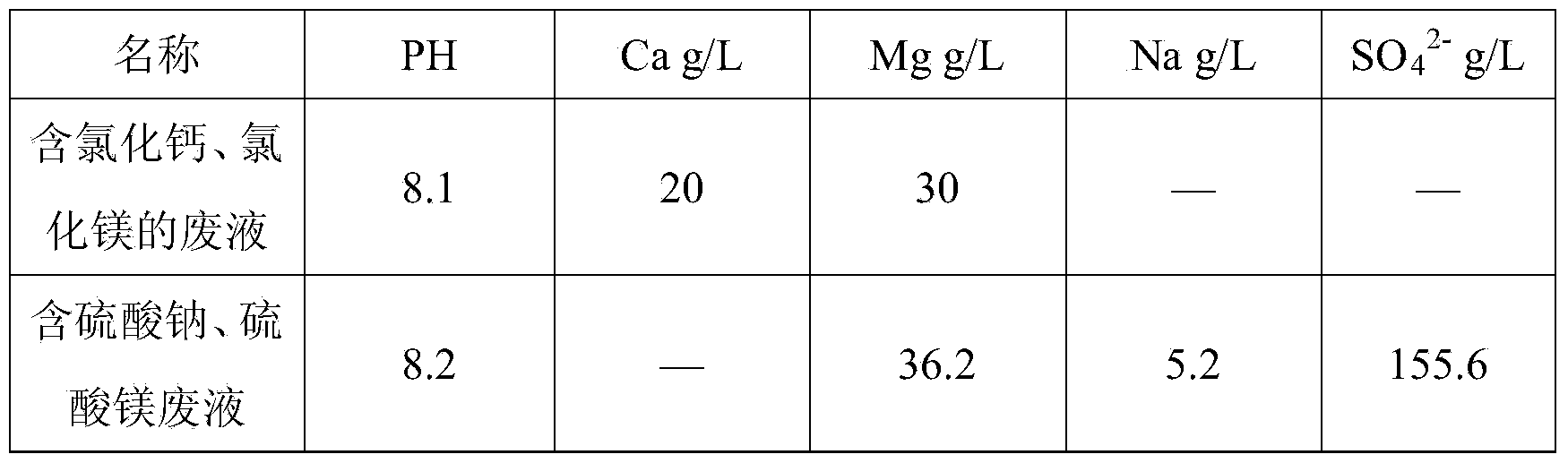A process of producing a snow melting agent from waste liquids obtained by subjecting laterite nickel ore to leaching with sulfuric acid and hydrochloric acid and to nickel precipitation
A technology of laterite nickel ore and sulfuric acid hydrochloric acid, applied in calcium/strontium/barium sulfate, chemical instruments and methods, magnesium chloride, etc., can solve the problems of high salt concentration in water, no market value, and difficult separation of mixed salts, etc. The effect of simple path, small investment and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Hydrochloric acid leaching of laterite nickel ore, iron removal with calcium carbonate, waste liquid containing calcium chloride and magnesium chloride after lime nickel precipitation and sulfuric acid leaching of laterite nickel ore, iron removal with calcium carbonate, sodium sulfate and magnesium sulfate after nickel precipitation with sodium hydroxide Waste liquid, its chemical composition is as follows:
[0018]
[0019] at 20m 3 Add 10m of waste liquid containing calcium chloride and magnesium chloride to the stirring tank 3 , add 4.94m of waste liquid containing sodium sulfate and magnesium sulfate 3 , 30°C, stirred for 30 minutes, a white calcium sulfate precipitate was generated, and the calcium precipitated material was obtained.
[0020] Filter the material after calcium precipitation with a filter area of 160m 2 Filtration by chamber filter press, the filter cakes are all flushed on the filter press for 2.3m 3 Wash, combine the filtrate and wash wat...
Embodiment 2
[0029] Hydrochloric acid leaching of laterite nickel ore, iron removal with calcium carbonate, waste liquid containing calcium chloride and magnesium chloride after lime nickel precipitation and sulfuric acid leaching of laterite nickel ore, iron removal with calcium carbonate, sodium sulfate and magnesium sulfate after nickel precipitation with sodium hydroxide Waste liquid, its chemical composition is as follows:
[0030]
[0031] at 20m 3 Add 10m of waste liquid containing calcium chloride and magnesium chloride to the stirring tank 3 , add 4.87m of waste liquid containing sodium sulfate and magnesium sulfate 3 , 5°C, stirred for 2 hours, a white calcium sulfate precipitate was generated, and the calcium precipitated material was obtained.
[0032] Filter the material after calcium precipitation with a filter area of 160m 2 Filtration by chamber filter press, the filter cakes are all flushed on the filter press for 2.3m 3 Wash, combine the filtrate and wash water. ...
Embodiment 3
[0042] Hydrochloric acid leaching of laterite nickel ore, iron removal with calcium carbonate, waste liquid containing calcium chloride and magnesium chloride after lime nickel precipitation and sulfuric acid leaching of laterite nickel ore, iron removal with calcium carbonate, sodium sulfate and magnesium sulfate after nickel precipitation with sodium hydroxide Waste liquid, its chemical composition is as follows:
[0043]
[0044] at 2m 3 Add 1m of waste liquid containing calcium chloride and magnesium chloride to the stirring tank 3 , add 0.31m waste liquid containing sodium sulfate and magnesium sulfate 3 , Stirring at 17°C for 1.5 hours, a white precipitate of calcium sulfate was generated, and the calcium precipitated material was obtained.
[0045] Filter the material after calcium precipitation with a filter area of 160m 2 The box filter press is filtered, and the filter cake is washed with 58 kg of water on the filter press, and the filtrate and washing water ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com