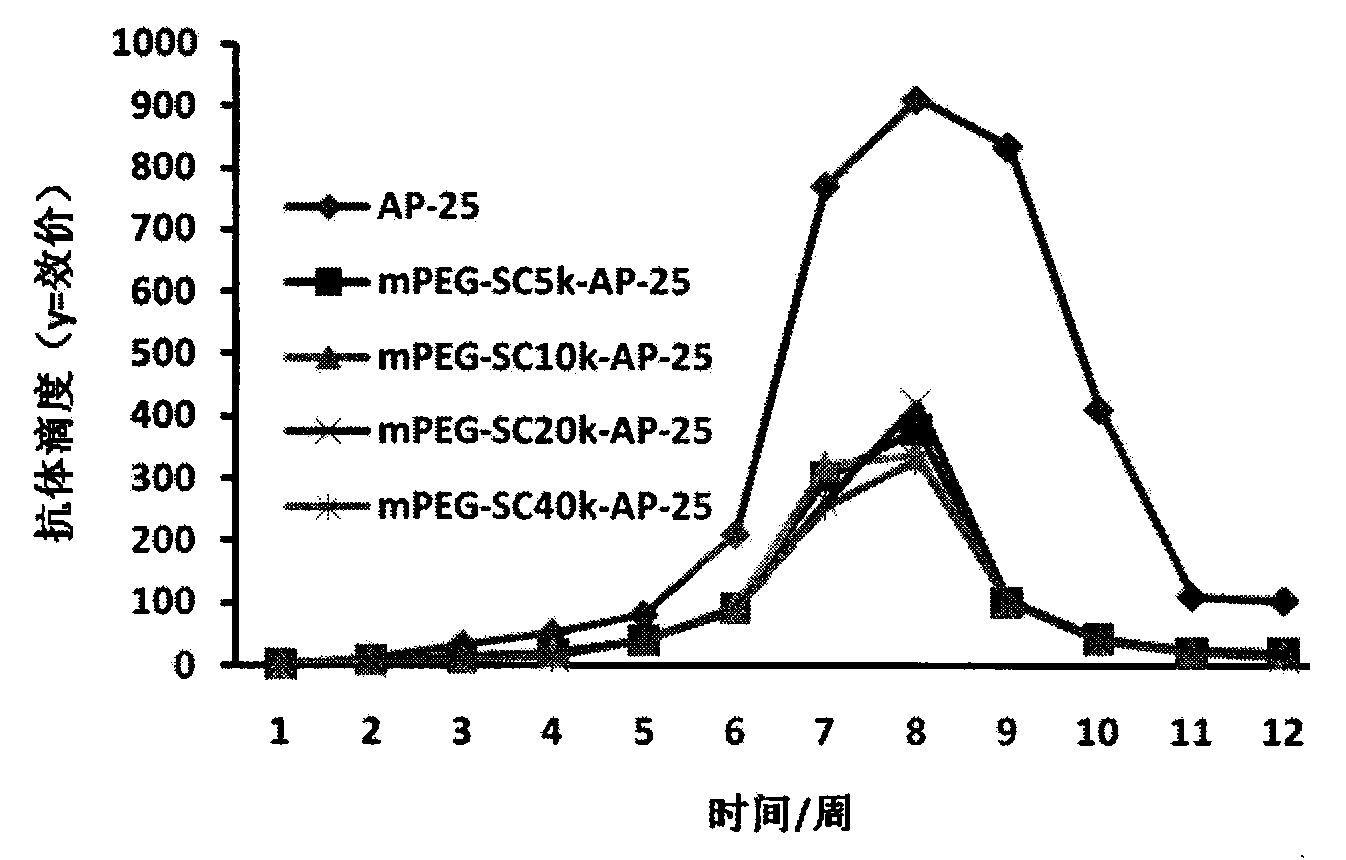
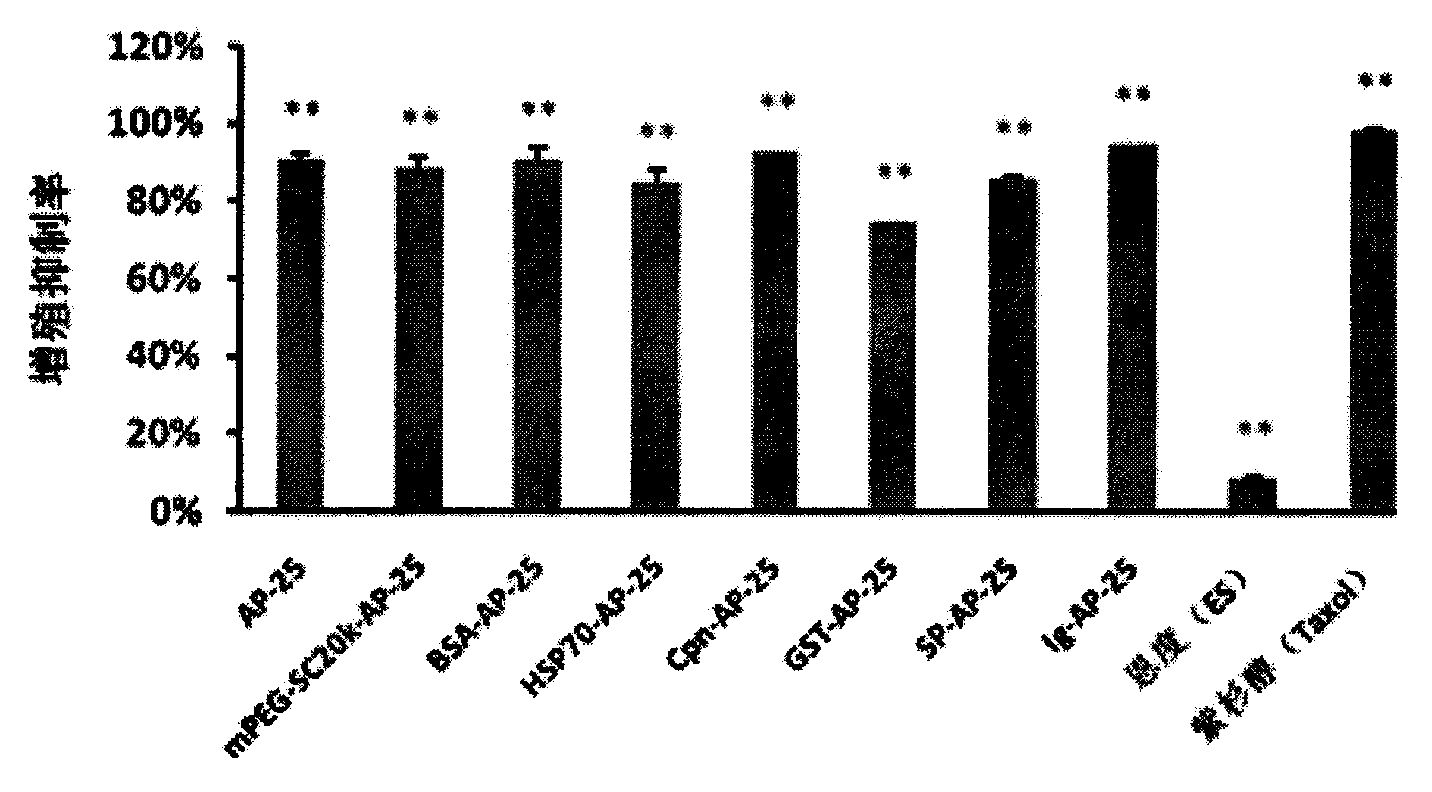
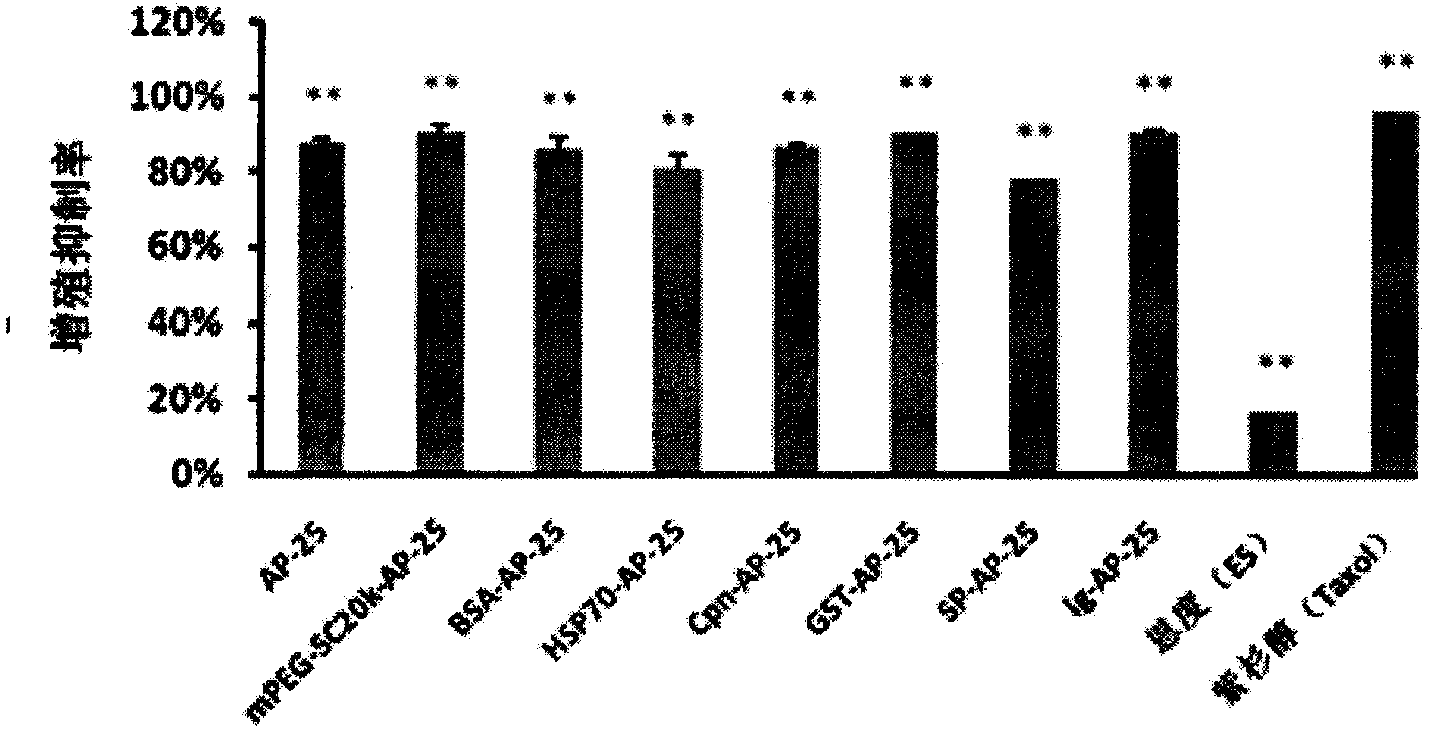
Integrin blocking agent AP-25 expressed by modification of polyethylene glycol and protein fusion and its application
An integrin blocker, AP-25 technology, applied to peptide/protein components, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve problems such as cumbersome process and difficulty in industrialization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] Example 1 Integrin blocking agent AP-25
[0084] Ala-Cys-Asp-Cys-Arg-Gly-Asp-Cys-Phe-Cys-Gly-Gly-Gly-Gly-Ile-Val-Arg-Arg-Ala-Asp-Arg-Ala-Ala-Val-Pro Solid-phase synthesis method, which uses Fmoc-Pro-2Cl resin as the starting material, and then uses protected amino acids to connect dipeptides to pentapentapeptides sequentially. After the peptide connection is completed, it is fully washed, and then peptides are cut and post-treated to obtain AP- 25 crude. Purify the crude product by first dissolving it, then using preparative high-performance liquid phase for two purifications, and finally concentrating and freeze-drying to obtain the pure product. Specific steps are as follows:
[0085] 1. Synthesis:
[0086] Weigh 1g of Fmoc-Pro-2Cl resin, pour it into a 1L glass sand core reaction column, add CH 2 Cl 2 10mi to fully expand the resin.
[0087] Uncapping: add 10ml of hexahydropyridine / DMF decapping solution, seal it and place it in a shaker for 5 minutes, the temp...
Embodiment 2
[0112] Synthesis of PEG
[0113] PEG is commissioned to be synthesized by Shanghai Jill Biochemical Co., Ltd., and PEG has four molecular weights of 5000D, 10000D, 20000D and 40000D.
Embodiment 3
[0114] Example 3 Steps for Polyethylene Glycol Modification of Polypeptides
[0115] mPEG-SC 20K Reaction with AP-25
[0116] Weigh 2g mPEG-SC respectively 20K Put 168.33mg AP-25 (molar ratio is 1.5:1) in 40ml-100ml prepared PBS buffer solution with pH5-8.5, overnight at 4°C, make it react, the PEG modification site is N-terminal Alanine, PEG reacts with free amino groups on alanine. PEG-SC500-20000 can be linked according to Example 2 to synthesize modified polypeptides.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com