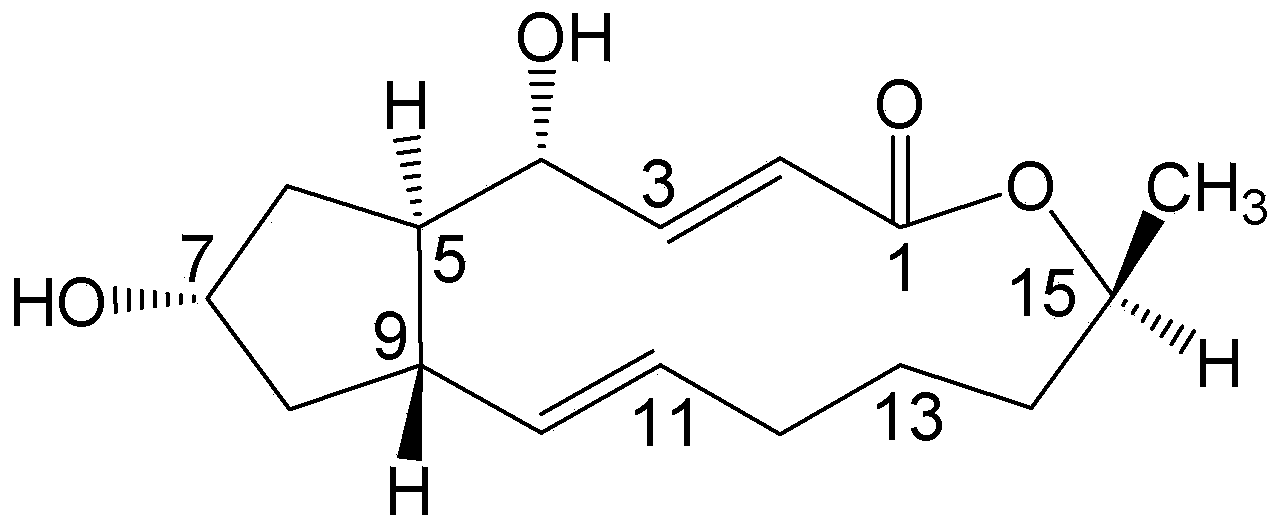
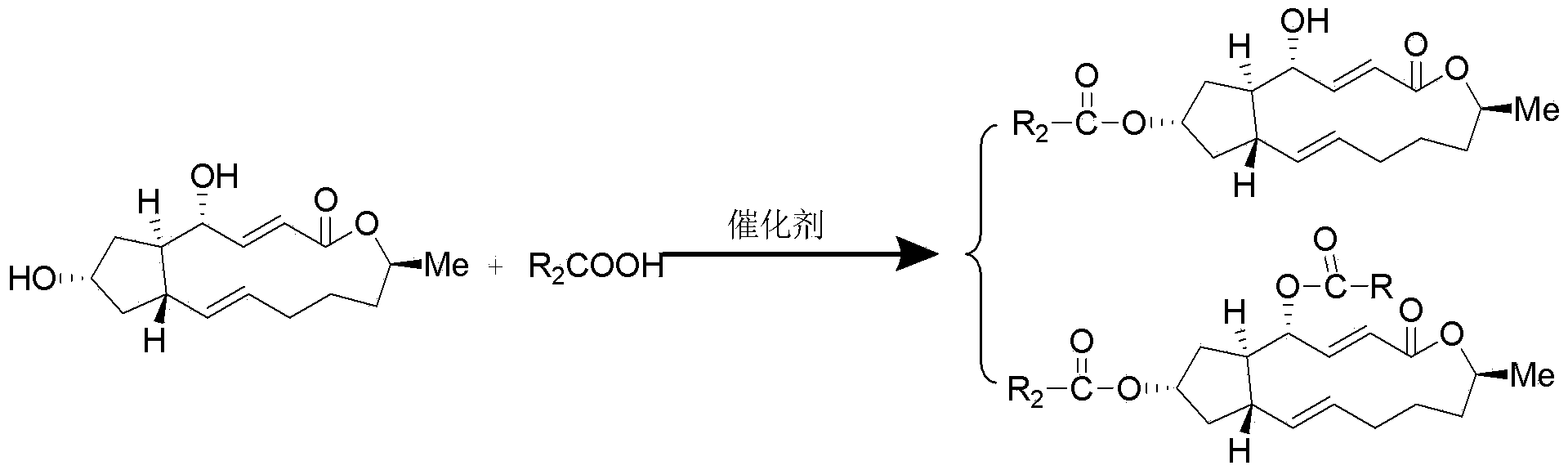
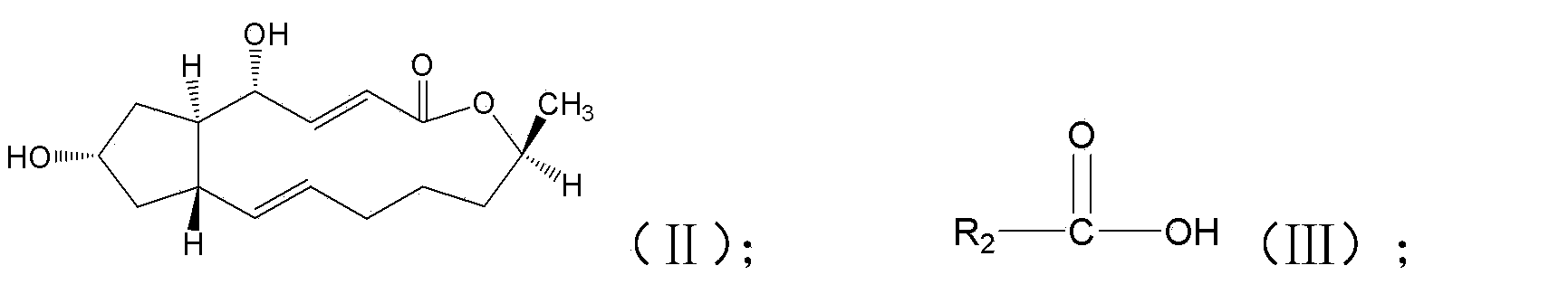Brefeldin A ester derivatives and their preparation method and use
A technology of brefeldia and feldspar, which is applied in the direction of drug combinations, medical preparations containing active ingredients, organic active ingredients, etc., can solve the problems of poor water solubility, unsatisfactory, and inability to apply anti-tumor therapeutic reagents, etc. Achieve the effect of simple method and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1: Preparation of 7-O-acetyl-BFA (I-1), 4,7-O-diacetyl-BFA (I-2)
[0035]
[0036] Add 10mL of anhydrous dichloromethane (10ml) to a 50mL round bottom flask with a magnetic stirring bar, then add BFA (100mg, 0.36mmol) and start stirring; then add acetic acid (45mg, 0.75mmol), EDC.HCl (1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride) (205mg, 108mmol), DMAP (4-dimethylaminopyridine) (22mg, 0.18mmol), at 40°C The reaction was heated for 24h to terminate the reaction. The reaction solution was diluted with 10ml of dichloromethane, washed with water (2×10ml), washed with saturated sodium chloride solution (2×10ml), the combined aqueous phase was extracted with ethyl acetate (1×30ml), the combined organic phase was anhydrous Dried over sodium sulfate, filtered, and concentrated to obtain a crude product, which was separated by thin-layer chromatography under the condition of developing solvent E / P=1:5 to obtain compound (I-1) (Rf=0.2, yield 42.50%) and ...
Embodiment 2
[0040] Example 2: Preparation of 7-O-(benzoic acid)acyl-BFA (I-3), 4,7-O-bis(benzoic acid)acyl-BFA (I-4)
[0041]
[0042] Add 10mL of anhydrous dichloromethane (10ml) to a 50mL round bottom flask with a magnetic stirring bar, then add BFA (100mg, 0.36mmol) and start stirring; then add benzoic acid (91.5mg, 0.75mmol), EDC .HCl (205mg, 108mmol), DMAP (22mg, 0.18mmol), heated at 40°C for 24h to terminate the reaction. The reaction solution was diluted with 10ml of dichloromethane, washed with water (2×10ml), washed with saturated sodium chloride solution (2×10ml), the combined aqueous phase was extracted with ethyl acetate (1×30ml), the combined organic phase was anhydrous Dried over sodium sulfate, filtered, and concentrated to obtain a crude product, which was separated by thin-layer chromatography under the condition of developing solvent E / P=1:5 to obtain compound (Ⅰ-3) (Rf=0.2, yield 46.7%) and Compound (I-4) (Rf=0.8 yield, 29.6%).
[0043] Compound Characterization: ...
Embodiment 3
[0046] Example 3: Preparation of 7-O-(p-fluorobenzoic acid)acyl-BFA (I-5), 4,7-O-bis(p-fluorobenzoic acid)acyl-BFA (I-6)
[0047]
[0048] Add 10mL of anhydrous dichloromethane (10ml) to a 50mL round bottom flask with a magnetic stirring bar, then add BFA (100mg, 0.36mmol) and start stirring; then add 4-fluorobenzoic acid (105mg, 0.75mmol) , EDC.HCl (205mg, 108mmol), DMAP (22mg, 0.18mmol), heated at 40°C for 24h to terminate the reaction. The reaction solution was diluted with 10ml of dichloromethane, washed with water (2×10ml), washed with saturated sodium chloride solution (2×10ml), the combined aqueous phase was extracted with ethyl acetate (1×30ml), the combined organic phase was anhydrous Dried over sodium sulfate, filtered, and concentrated to obtain a crude product, which was separated by thin-layer chromatography under the condition of developing solvent E / P=1:5 to obtain compound (Ⅰ-5) (Rf=0.2, yield 36.4%) and Compound (I-6) (Rf = 0.8, yield 20.0%).
[0049] Com...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com