Bone repair regeneration material and preparation method thereof
A technology for bone repair and composite scaffolds, applied in medical science, prosthesis, etc., can solve the problems of loss of mechanical support, low molecular weight, and decreased mechanical properties of composite materials with hydroxyapatite content, and achieves adjustable pore size and porosity. The effect of uniform distribution of components and excellent biological function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0044] The preparation method of the above-mentioned bone repair regeneration material of the present invention comprises the following 5 steps:
[0045] (1) Preparation of calcium phosphate salt composition
[0046] Prepare the powder of the calcium phosphate salt composition, wherein the content of hydroxyapatite is 50-70wt%, and the content of β-tricalcium phosphate is 30-50wt%; preferably, the powder of the calcium phosphate salt composition Prepared by in-situ mixing method, comprising the following steps:
[0047] (1.1) Weigh the calculated amount of calcium nitrate Ca(NO 3 ) 2 and trimethyl phosphate (CH 3 O) 3 PO, dissolved in distilled water and ethanol, respectively;
[0048] (1.2) Then add citric acid C in an equimolar amount to calcium ions in the calcium nitrate aqueous solution 6 h 8 o 7 After fully dissolving, mix the calcium nitrate aqueous solution and the trimethyl phosphate alcohol solution, adjust the pH of the mixed solution to 3-11 with ammonia wa...
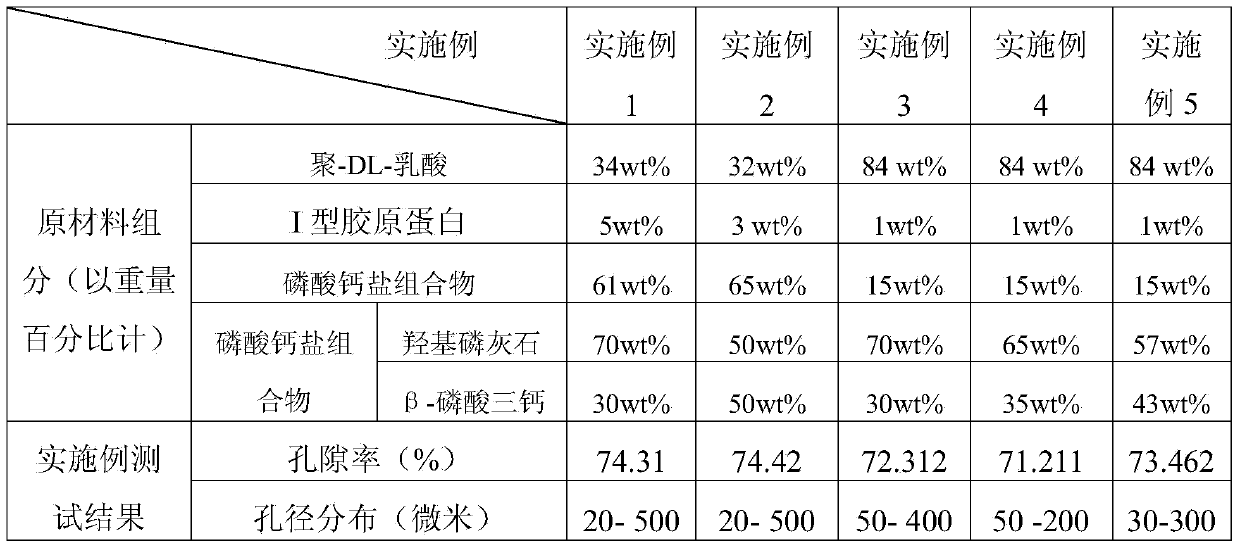
Embodiment 1
[0066] A bone repair regeneration material of the present invention is prepared by the following method:
[0067] (1) Preparation of calcium phosphate salt composition
[0068](1.1) Weigh 13.702g (0.0835mol) of calcium nitrate and 7.05g (0.05mol) of trimethyl phosphate according to the Ca / P molar ratio of 1.67, and dissolve them in 80ml of distilled water and 30ml of ethanol respectively;
[0069] (1.2) Add 16.032g of citric acid in an equimolar amount to calcium ions in the calcium nitrate aqueous solution. After fully dissolving, mix the calcium nitrate aqueous solution and the ethanol solution of trimethyl phosphate, and adjust the pH of the mixture to 7.5 with ammonia water. , aging at room temperature for 48 hours; then, drying at 190°C for 2 hours to generate a xerogel;
[0070] (1.3) Calcining the xerogel obtained in step (1.2) at 500°C for 1 hour to obtain the desired nanopowder of calcium phosphate salt composition, wherein the mass percentage of hydroxyapatite is 70...
Embodiment 2
[0084] A bone repair regeneration material of the present invention is prepared by the following method:
[0085] (1) Preparation of calcium phosphate salt composition
[0086] (1.1) Weigh 12.307g (0.075mol) of calcium nitrate and 7.05g (0.05mol) of trimethyl phosphate according to the Ca / P molar ratio of 1.5, and dissolve them in 80ml of distilled water and 30ml of ethanol respectively;
[0087] (1.2) Add 14.400g of citric acid in an equimolar amount to calcium ions in the calcium nitrate aqueous solution. After fully dissolving, mix the calcium nitrate aqueous solution and trimethyl phosphate alcohol solution, and adjust the pH of the mixture to 11 with ammonia water. Aging at room temperature for 48 hours; then, drying at 150°C for 3 hours to generate a xerogel;
[0088] (1.3) Calcining the xerogel obtained in step (1.2) at 600°C for 2 hours to obtain the desired nano-powder of calcium phosphate salt composition, wherein the mass percentage of hydroxyapatite is 50%, β - T...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com