Organic fluorochrome molecule and synthesis method and application thereof
A technology of fluorescent dyes and synthesis methods, applied in the field of organic fluorescent dye molecules and their synthesis, and nanoparticles used in the fluorescent dye field of living cells, can solve the problems of poor modifiability, high price, few types, etc., and achieves good stability, The effect of cheap raw materials and convenient preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] The synthetic route is as follows:
[0045]
[0046] In the formula: Me is methyl.
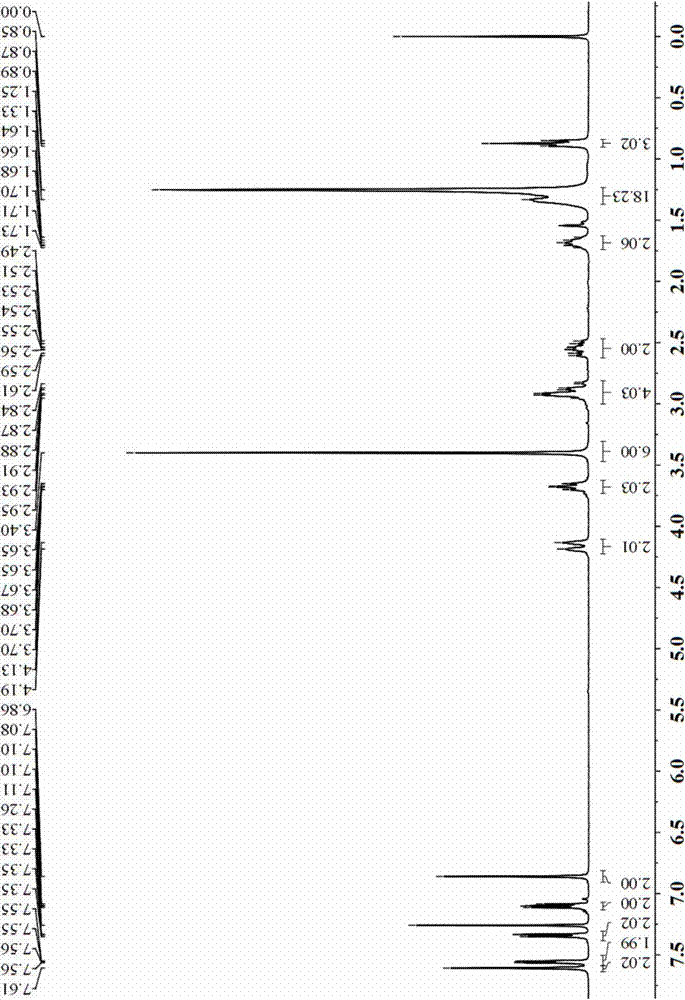
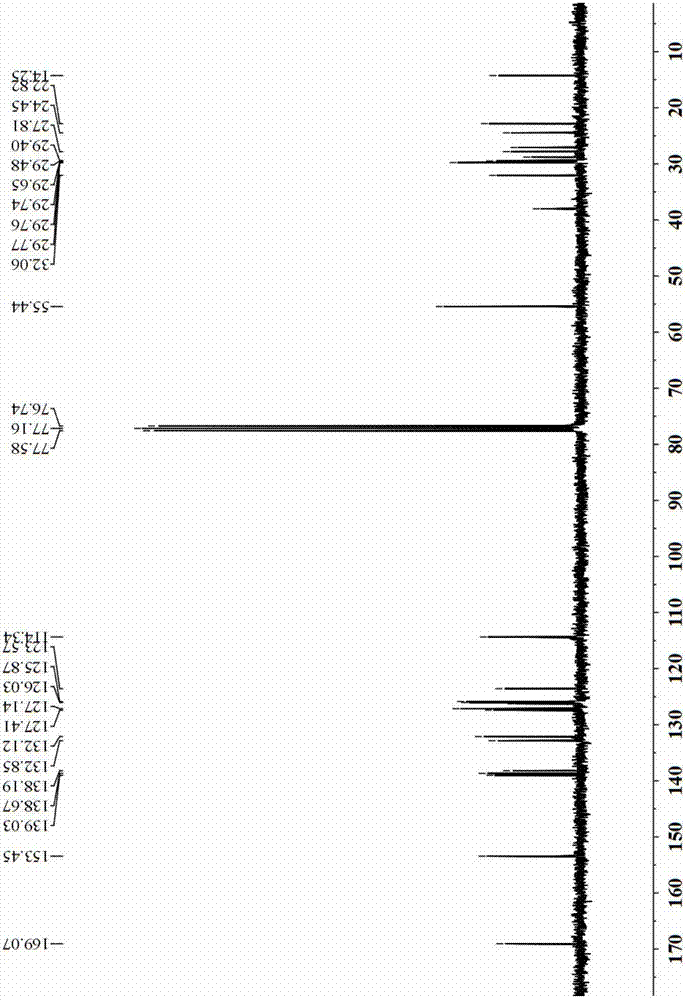
[0047] 1) Add 57g of raw material (2',3"-dibromo-1,2,7,8-tetrahydro-2",3'-dimethoxy-3,4:5,6 into a 2500ml round bottom flask -Dibenzophenanthrene-9,10-dicarboxylic acid anhydride) and 59g n-dodecylamine, and add 100ml DMF, react at 70℃ for 24 hours, then spin dry the reaction solution and wash with dichloromethane , Obtain 42.6g lactimide product, yield 70%;
[0048] 2) Take 740 mg of the lactimide product obtained in step 1), 384 mg of 2-thiophene boronic acid and 424 mg of Na 2 CO 3 Add to a 25ml two-neck flask, add 50ml of toluene, 30ml of ethanol and 20ml of aqueous solution with a syringe under the protection of argon. After 5 minutes of argon, add 50mg of the catalyst tetrakis(triphenylphosphine) palladium. The heating reaction is carried out under the heating temperature of 90~110℃, and the reaction is 12 hours. After the reaction is completed, the organic layer is taken and MgSO 4 Dr...
Embodiment 2
[0060] The synthetic route is as follows:
[0061]
[0062] In the formula: Me is methyl.
[0063] 1) Add 57g of raw material (2',3"-dibromo-1,2,7,8-tetrahydro-2",3'-dimethoxy-3,4:5,6 into a 2500ml round bottom flask -Dibenzophenanthrene-9,10-dicarboxylic acid anhydride) and 59g n-dodecylamine, and add 100ml DMF, react at 70℃ for 24 hours, then spin dry the reaction solution and wash with dichloromethane , Obtain 42.6g lactimide product, yield 70%;
[0064] 2) Take 740 mg of the lactimide product obtained in step 1), 1130 mg of 5-diphenylamino-2-thiophene boronic acid and 424 mg of Na 2 CO 3 Add to a 25ml two-neck flask, add 5ml of toluene, 3ml of ethanol and 2ml of aqueous solution with a syringe under argon protection, add 50mg of catalyst tetrakis(triphenylphosphine) palladium after 5 minutes of argon protection, under argon protection Reflux the reaction for 12 hours, after the completion of the reaction, take the organic layer and use MgSO 4 Dry, filter, spin-dry, and separate...
Embodiment 3
[0077] The aqueous solution of nanoparticles containing 2-thienyl-substituted organic fluorescent dye molecules of Example 1 and the aqueous solution of nanoparticles containing 5-diphenylamino-2-thienyl-substituted organic fluorescent dye molecules of Example 2 were performed Ultraviolet-visible absorption spectroscopy and fluorescence spectroscopy were measured, and the optical property data are shown in Table 1.
[0078] Table 1.
[0079]
[0080] Note: The quantum yield is the absolute quantum yield in aqueous solution.
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com