Ester-type curable compound, composition containing same, cured product, and method for producing ester-type curable compound
A production method and compound technology, which are applied in the production field of ester curable compounds, and can solve the problem that heat resistance and flexibility cannot be satisfied at the same time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
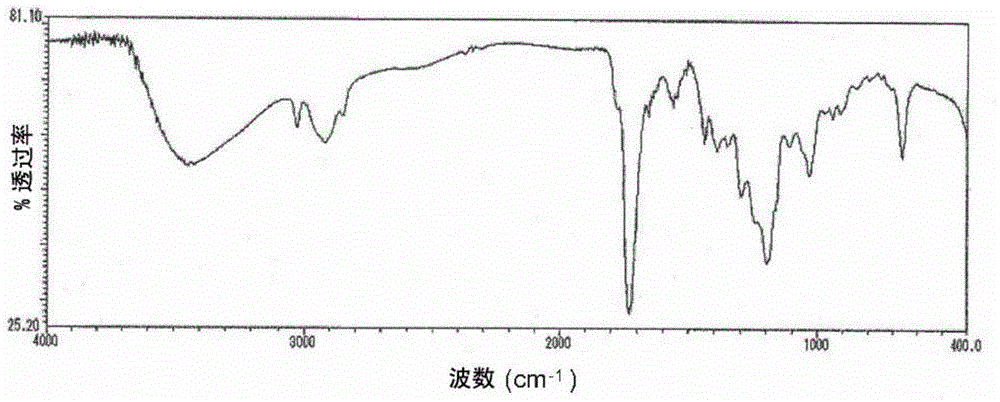
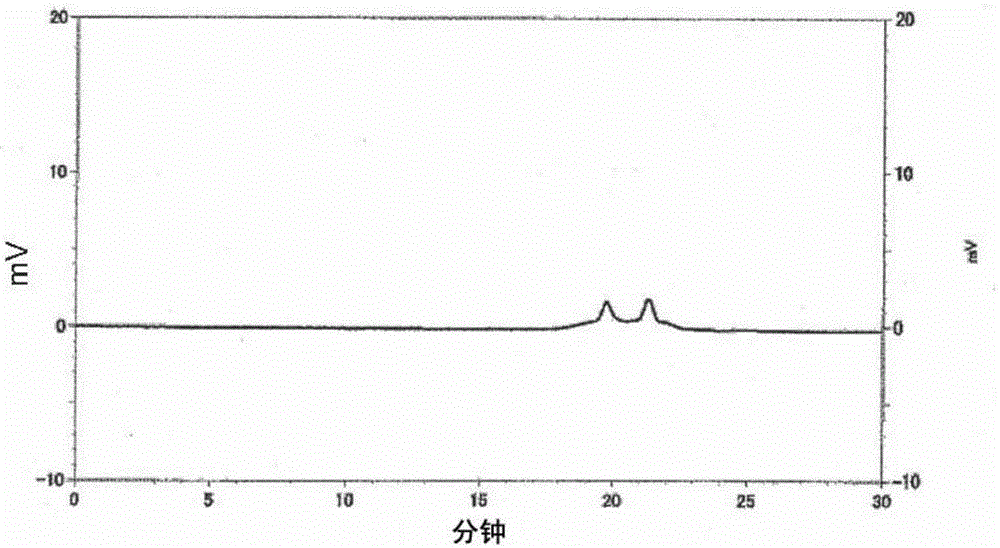
[0102] Add 152 parts of tetrahydrophthalic anhydride, 74 parts of glycidol, 97 parts of diethylene glycol monoethyl ether acetate, and 0.226 parts of three Phenylphosphine was reacted at 70 to 80° C. for 6 hours to obtain a reaction solution containing a compound having an acid value of 167 mgKOH / g and an epoxy equivalent of 341 g / eq. It corresponds to n≈0.5 of the aforementioned general formula (1), that is, a mixture of compound (k1) and compound (k2). The infrared absorption spectrum (infrared absorption spectrometry (hereinafter referred to as "IR spectrum")) of the obtained compound is shown in figure 1 , the chromatogram obtained by gel permeation chromatography is shown in figure 2 . Then, add 202 parts of cresol novolac type epoxy resins (epoxy equivalent 202g / eq.), 190 parts of diethylene glycol monoethyl ether acetate, and 1.1 parts of triphenylphosphine in this reaction solution, at 90~ The reaction was carried out at 100° C. for 10 hours to obtain a reaction s...
Embodiment 2
[0115] 323.226 parts of the mixture solution of compound (k1) and compound (k2) obtained in Example 1, 47.7 parts of acrylic acid, 21 parts of diethylene diethylene glycol, Alcohol monoethyl ether acetate and 0.87 parts of triphenylphosphine were reacted at 90 to 100° C. for 8 hours while blowing air in, to obtain a reaction solution with 70% of non-volatile content. The acid value of the ester-type curable compound thus obtained was 197 mgKOH / g.
[0116] Then, 143 parts of the reaction solution obtained, 106 parts of cresol novolak type epoxy resin (epoxy equivalent 202g / eq.), 1 part of dicyandiamide, 25 parts of trimethylolpropane trimethacrylate, and 10 parts of 2-methyl-1-[4-(methylthio)phenyl]-2-morpholinopropan-1-one were kneaded to obtain a photo- and heat-curable composition. The composition is coated on a polyester film with a thickness of 50-60 μm, and the coating film is dried in a hot air circulation dryer at 80°C for 30 minutes, and then heated at 500mJ / cm 2 It ...
Embodiment 3
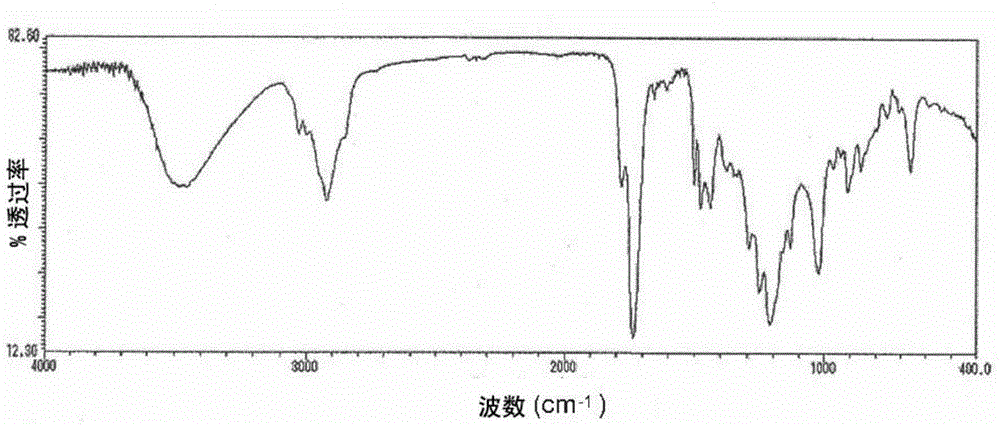
[0118] 392.796 parts and 76 parts of the ester-type curable compound solution obtained in Example 2 with 70% non-volatile components and an acid value of 197 mgKOH / g were added to a reaction container equipped with a thermometer, a stirrer, a reflux condenser tube, and an air blowing tube. A reaction in which diethylene glycol monoethyl ether acetate and 16.4 parts of 2,2'-azobisisobutyronitrile were reacted at 70-80°C for 8 hours while blowing nitrogen gas to obtain 60% of non-volatile components solution. The acid value of the ester-type curable compound thus obtained was 195 mgKOH / g, and Mw was 14,700. The IR spectrum of the obtained ester type curable compound is shown in Figure 4 .
[0119] Next, 167 parts of the obtained reaction solution, 105 parts of cresol novolak type epoxy resins (202 g / eq. of epoxy equivalent), and 1 part of dicyandiamide were kneaded, and the thermosetting composition was obtained. Then, it carried out similarly to Example 1, the test film and...
PUM
| Property | Measurement | Unit |
|---|---|---|
| acid value | aaaaa | aaaaa |
| acid value | aaaaa | aaaaa |
| epoxy equivalent | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com