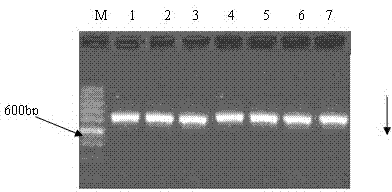
Establishment of levofloxacin-induced Shigella drug-resistance gene mutation time sequence models
A levofloxacin and sequential model technology, applied in the biological field, can solve the problems that the order of quinolone-resistant drug target gene mutations cannot exclude its own random mutations, and cannot explain the problem, so as to achieve the effect of eliminating its own random mutations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073]Embodiment 1: The establishment of levofloxacin-induced Shigella drug-resistant gene mutation time series model: 1. To identify, preserve Shigella sonnei, carry out cefazolin, cefotaxime, ampicillin, gentamicin, chloramphenicol Antibiotic susceptibility test of 12 kinds of antibiotics, including tetracycline, cotrimoxazole, nalidixic acid, pipemidic acid, ciprofloxacin, norfloxacin and levofloxacin, with the quality control strain as the control, measuring with ruler including drug susceptibility The diameter of the inner antibacterial ring of the paper is limited to the limit of no obvious bacterial growth with the naked eye as the edge of the antibacterial ring, and the guidelines established by the National Committee for Clinical Laboratory Standards (NCCLS) are used to determine the sensitivity of the bacteria , Moderately sensitive or resistant. Through the above drug sensitivity test on the identified and preserved Shigella sonnei, it was verified that it was sensi...
Embodiment 2
[0100] Example 2: Establishment of a time-series model of levofloxacin-induced Shigella drug-resistant gene mutation: the method steps are the same as in Example 1, wherein the bacterial solid medium is LB plate, the bacterial liquid medium is LB liquid culture, and the temperature in step (4) is 32°C Bacteria were cultured with shaking until OD600=1 in the logarithmic phase. Take a certain amount of bacterial liquid in step (4) to 5 tubes of LB liquid medium with a levofloxacin content of 0.03125 μg / ml for another generation, and then take a certain amount of bacterial liquid to LB liquid medium with a levofloxacin concentration of 0.0625 to pass two tubes. Generation, before the drug concentration reached the levofloxacin sensitive and intermediate critical value, the concentration of levofloxacin was doubled in turn to culture the bacteria, and when the drug concentration reached the levofloxacin sensitive and intermediate critical value, the concentration of levofloxacin wa...
Embodiment 3
[0104] Example 3 Establishment of a time-series model of levofloxacin-induced Shigella drug-resistant gene mutation: the method steps are the same as in Example 1, wherein the bacterial solid medium is LB plate, the bacterial liquid medium is LB liquid culture, and in step (4) shake at 39°C Bacteria were cultured and grown to log phase OD600=0.6. Take a certain amount of bacterial liquid in step (4) to 10 tubes of LB liquid medium with a levofloxacin content of 0.03125 μg / ml for another generation, and then take a certain amount of bacterial liquid to LB liquid medium with a levofloxacin concentration of 0.0625 for two passages. Generation, before the drug concentration reached the levofloxacin sensitive and intermediate critical value, the concentration of levofloxacin was doubled in turn to culture the bacteria, and when the drug concentration reached the levofloxacin sensitive and intermediate critical value, the concentration of levofloxacin was successively increased by...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com