Hydroxyl-terminated hyperbranched polyurethane-ester polymer as well as modified micro-fluidic chip and application thereof
A technology of terminal hydroxyl hyperbranching and microfluidic chip, which is applied in the preparation of organic compounds, cyanide reaction preparation, organic chemistry, etc., can solve the problem of separation peak tailing and broadening, separation efficiency and migration time repeatability decline, affecting organic Solve problems such as pesticide separation effect and microchannel surface inhomogeneity, achieve low cost, save money, and fast analysis speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
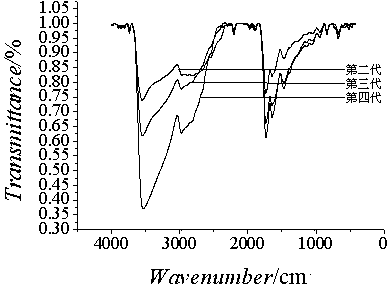
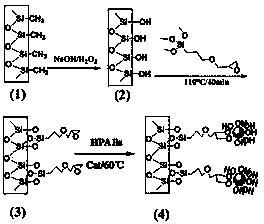
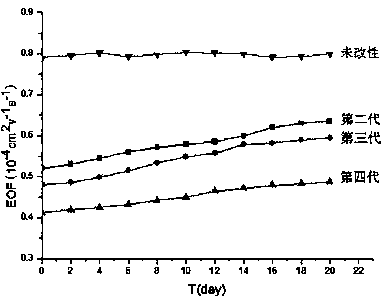
[0048] Example 1 Preparation of the second-generation hydroxyl-terminated hyperbranched polyamine-ester polymer
[0049] Nitrogen was passed into a 250 mL three-necked flask for 10 min, 26.638 g of diisopropanolamine, 17.218 g of methyl acrylate and 10 ml of methanol were mixed in a small beaker and then added to the three-necked flask, and the mixture was ventilated with nitrogen at room temperature for 10 min , stirred for 30 min and heated to 40 °C for 4 h, then distilled off methanol under reduced pressure to obtain a colorless and transparent oily AB 2 monomer, the reaction formula is as follows.
[0050]
[0051] Into a 250 mL four-necked bottle, nitrogen was passed for 10 min, and 9.8676 g of synthetic AB was added 2 Monomer, 0.0493 g of p-toluenesulfonic acid and 0.74595 g of triethanolamine, heated up to 85 °C, continued to flow nitrogen, stirred and reacted for 24 h, and then distilled under reduced pressure to remove methanol and unreacted small molec...
Embodiment 2
[0053] Example 2 Preparation of third-generation hydroxyl-terminated hyperbranched polyamine-ester polymers
[0054] In the 250 mL four-necked bottle, nitrogen was passed for 10 min, and 21.928 g of AB synthesized in Example 1 was added. 2 Monomer, 0.1096g of p-toluenesulfonic acid and 0.74595g of triethanolamine, heated up to 85°C, continued nitrogen flow, stirred and reacted for 24 hours, and then distilled under reduced pressure to remove methanol and unreacted small molecular compounds in the reaction to obtain the third Substituted hydroxyl-terminated hyperbranched polyamine-ester polymers. Among them, triethanolamine and AB 2 The molar ratio of the monomers is 1:21, and the structural formula of the third-generation hydroxyl-terminated hyperbranched polyamine-ester polymer is shown above.
Embodiment 3
[0055] Example 3 Preparation of the fourth-generation hydroxyl-terminated hyperbranched polyamine-ester polymer
[0056] In the 250 mL four-necked bottle, nitrogen was passed for 10 min, and 43.856 g of AB synthesized in Example 1 was added. 2 monomer, 0.2193g of p-toluenesulfonic acid and 0.74595g of triethanolamine, the temperature was raised to 85°C, nitrogen was continued to flow, and the reaction was stirred for 24 hours, and then methanol and unreacted small molecule compounds in the reaction were distilled off under reduced pressure to obtain the fourth Substituted hydroxyl-terminated hyperbranched polyamine-ester polymers. Among them, triethanolamine and AB 2 The molar ratio of the monomers is 1:45, and the structural formula of the fourth-generation hydroxyl-terminated hyperbranched polyamine-ester polymer is shown above.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com