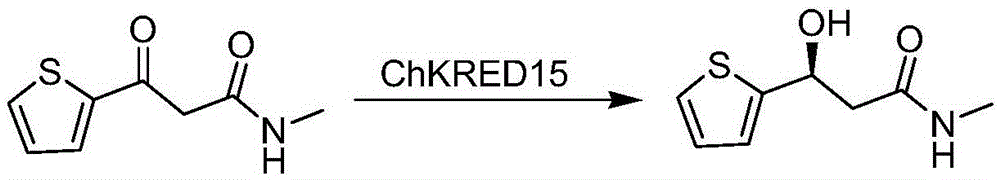Carbonyl reductase, its gene and its use in the preparation of duloxetine chiral intermediate
A reductase and carbonyl technology, applied in the field of biocatalyst production, can solve the problems of low optical purity, large waste discharge, difficult to use pharmaceutical intermediates, etc., and achieves simple conversion process, stable coenzyme circulation system, and large industrial application prospects. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Embodiment 1 Amplification of carbonyl reductase (ChKRED15) gene
[0019] According to the whole genome sequencing information of Chryseobacteriumsp.CA49, a large number of carbonyl reductases were excavated, one of which can catalyze the formation of (S)-N from N-methyl-3-oxo-3-(2-thienyl)propionamide - The enzyme with the function of methyl-3-hydroxy-3-(2-thienyl)propanamide is the carbonyl reductase ChKRED15 involved in the present invention, and the gene acquisition process is a conventional operation method. Design the following primers: Forward: 5′-GCG GAATTC ATGAAAACAGTATTAATTACAGGCGCC-3′ (restriction site: EcoRI); reverse: 5′-GCG AAGCTT CTACCACGGACTGATTCCGG-3′ (restriction site: HindIII), using the genomic DNA of Chryseobacterium aureus CCTCCM2012484 as a template, was amplified by PCR to obtain the gene of carbonyl reductase ChKRED15.
[0020] The PCR program was: pre-denaturation at 95°C for 5 min, denaturation at 94°C for 30 s, annealing at 55°C for 30 s, ...
Embodiment 2
[0023] Example 2 Induced expression of carbonyl reductase (ChKRED15)
[0024]Pick the single clone of the recombinant E.coli (pET28a-ChKRED15) constructed in Example 1, inoculate it in LB medium containing 50 μg / mL kanamycin, and culture it at 37°C and 180 rpm for 16 hours as a seed solution. % inoculum rate was transferred to fresh LB or TB medium (500mL capacity shake flask liquid 200mL medium), cultured with shaking at 37°C for 4h, and then added IPTG with a final concentration of 0.5mM to induce the expression of carbonyl reductase ChKRED15 gene. After induction at 30°C for 36h, cells were harvested by refrigerated centrifugation at 8000rpm for 10min at 4°C.
[0025] Bacteria can be used as biocatalysts or for protein purification.
Embodiment 3
[0026] Example 3 Separation and Purification of Carbonyl Reductase (ChKRED15)
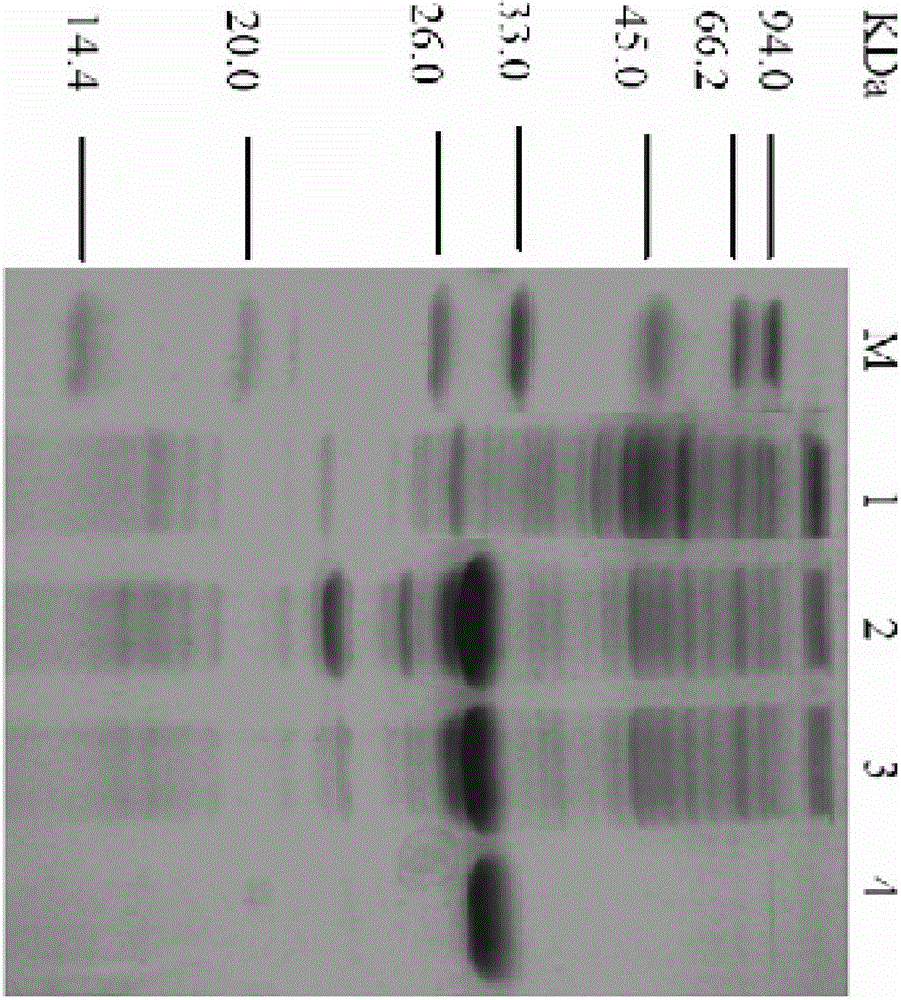
[0027] The bacteria obtained in Example 2 were resuspended in the binding buffer (100mM, pH8.0 sodium phosphate buffer, containing 300mMNaCl, 5mM imidazole), crushed by a high-pressure homogenizer, centrifuged at 12000rpm for 15min, and the supernatant was mixed with the above-mentioned After incubation with the Ni affinity chromatography resin equilibrated with the binding solution, use the washing buffer (100mM, pH8.0 sodium phosphate buffer, containing 300mM NaCl, 10mM imidazole) to rinse until there is basically no foreign protein, and then wash with the elution buffer (100mM , pH8.0 sodium phosphate buffer, containing 300mM NaCl, 250mM imidazole) to elute and collect the target protein, after electrophoresis to identify the purity, combine the target protein and dialyze with dialysis buffer (100mM, pH8.0 potassium phosphate buffer) for 48h, ultrafiltration After concentration, the protein conc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com