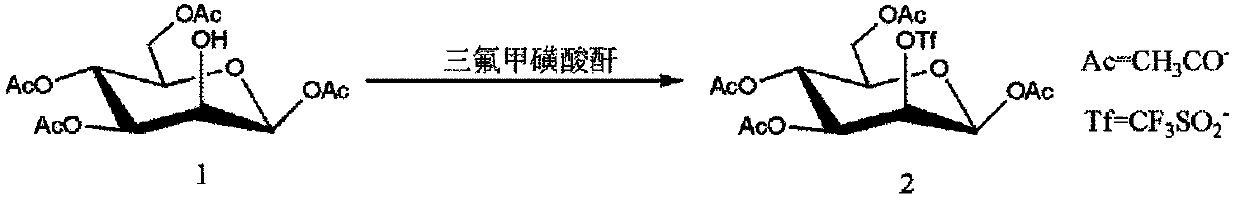
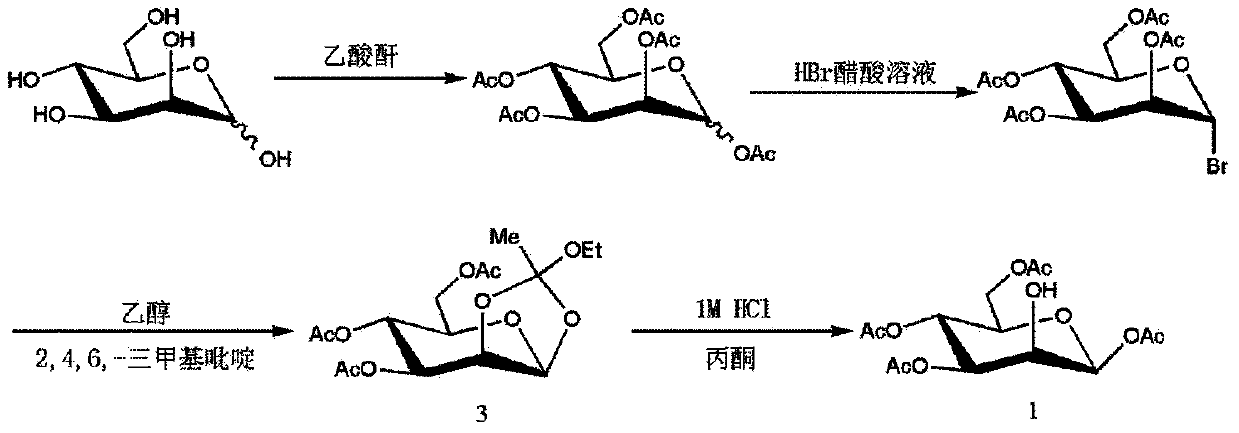
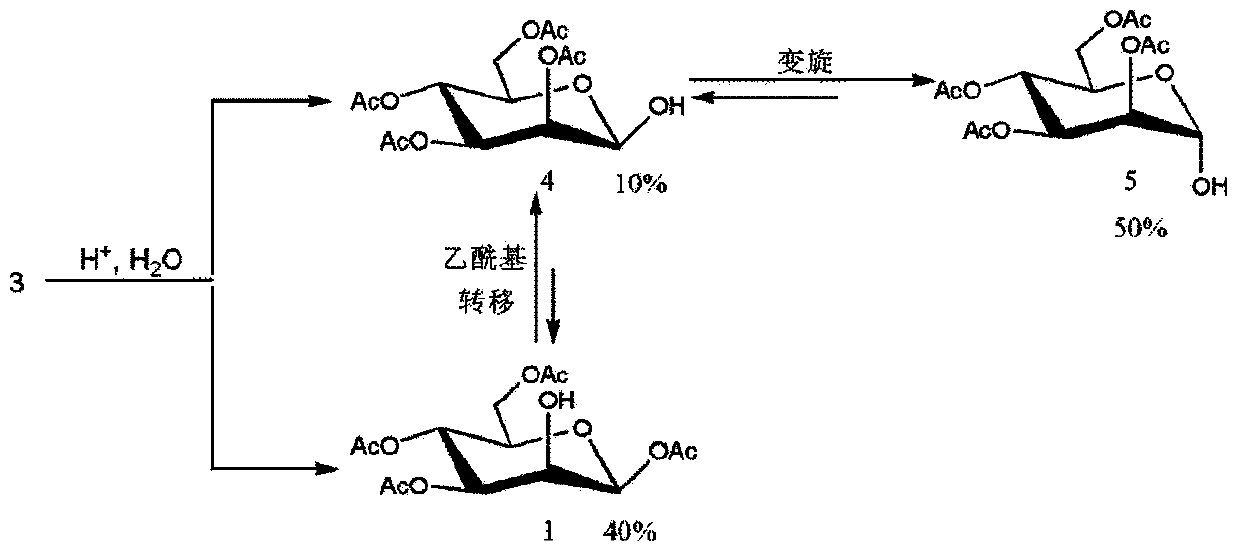
Method for purifying 1,3,4,6-tetra-O-acetyl-2-O-triflat-beta-D-mannopyranose intermediate
A technology of trifluoromannose and purification method, applied in the field of purification of trifluoromannose intermediate, 1,3,4,6-tetraacetyl-β-D-mannose, can solve the problem of yield and purity. High, affecting the yield and purity of trifluoromannose, to achieve the effect of good stability and long shelf life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The purification method of trifluoromannose intermediate 1,3,4,6-tetraacetyl-β-D-mannose described in this example comprises the following steps:
[0032] Add 50mL of the mixed liquid containing n-butanol and ethylene glycol monomethyl ether in a three-necked flask, the ratio of the volume of n-butanol and ethylene glycol monomethyl ether is 6:1, add the above-mentioned 1,3, 4,6-tetraacetyl-β-D-mannose crude product 10g, and the solution was heated to 80 ° C until the 1,3,4,6-tetraacetyl-β-D-mannose crude product was completely dissolved, then The temperature of the above solution was lowered to 8°C, and allowed to stand for crystallization for 1 hour, and the solution of the above crystallization was filtered, and 3 mL of n-butanol solution was added to wash the above filtered crystals, and the washing was repeated for 2-3 times, and the washed crystals were placed in vacuum at 45°C. After drying for 8 hours, 1,3,4,6-tetraacetyl-β-D-mannose crystals were obtained.
Embodiment 2
[0034] The purification method of trifluoromannose intermediate 1,3,4,6-tetraacetyl-β-D-mannose described in this example comprises the following steps:
[0035] Add 100mL of the mixed liquid containing n-butanol and ethylene glycol monomethyl ether in a three-necked flask, the ratio of the volume of n-butanol and ethylene glycol monomethyl ether is 6:1, add the above-mentioned 1,3, 10 g of crude 4,6-tetraacetyl-β-D-mannose, and heating the solution to 100° C. until the crude 1,3,4,6-tetraacetyl-β-D-mannose is completely dissolved, Subsequently, the above-mentioned dissolving solution was cooled to 10° C., and allowed to stand for crystallization for 2 hours. The above-mentioned crystallization solution was filtered, and 5 mL of n-butanol solution was added to wash the above-mentioned filtered crystals, and the washing was repeated for 2-3 times. °C for 10 hours under vacuum to obtain the 1,3,4,6-tetraacetyl-β-D-mannose crystals.
Embodiment 3
[0037] The purification method of trifluoromannose intermediate 1,3,4,6-tetraacetyl-β-D-mannose described in this example comprises the following steps:
[0038] Add 150mL of the mixed liquid containing n-butanol and ethylene glycol monomethyl ether in a three-necked flask, the volume ratio of n-butanol and ethylene glycol monomethyl ether is 6:1, add the above prepared 1,3, 10 g of crude 4,6-tetraacetyl-β-D-mannose, and heating the solution to 110° C. until the crude 1,3,4,6-tetraacetyl-β-D-mannose is completely dissolved, Subsequently, the above-mentioned solution was cooled to 15° C., and allowed to stand for crystallization for 3 hours, the solution of the above-mentioned crystal was filtered, and 7 mL of n-butanol solution was added to wash the above-mentioned filtered crystal, and the washing was repeated for 2-3 times, and the washed crystal was placed at 55 °C for 12 hours under vacuum to obtain the 1,3,4,6-tetraacetyl-β-D-mannose crystals.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com