Fenneropenaeus chinensiss anti-lipopolysaccharide factor as well as preparation and application thereof
A technology for anti-lipopolysaccharide factor and penaeus prawn, applied in the field of genetic engineering, can solve the problems of mutated pathogenic microorganisms, increased resistance of pathogenic organisms, lack of knowledge about drug use by farmers, and achieves strong growth inhibitory effect and obvious sterilization. effect, significant growth inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Chinese prawn anti-lipopolysaccharide factor mature peptide FcALF, as shown in the amino acid sequence of SEQ ID NO.1,
[0036] QGWEAVAAAVAVKIVGLWRNEKTELLGHECKFTVKPYIKRFQLYYKGRMWCPGWTAIRGEAKTRSRSGVAGRTAKDFVRKAFQQGLISQQQANQWLNS
[0037] The nucleotide sequence of the gene is shown as SEQ No.2, and its 5' end and 3' end have start codon (ATG) and stop codon (TAG) respectively.
[0038] ATGCGAGTTTCCGTGTTGGCAAGCCTGGTGCTGGTGGTGTCCCTGGTGGCACTCTTCGCCCCGCAGTGCCAGGCTCAAGGGTGGGAGGCTGTGGCAGCGGCCGTCGCCGTCAAGATTGTTGGGCTGTGGAGGAACGAGAAAACCGAACTCCTCGGCCACGAGTGCAAGTTCACCGTCAAGCCTTACATTAAGAGGTTCCAGTTGTACTACAAGGGGAGGATGTGGTGCCCAGGCTGGACGGCCATCAGAGGAGAAGCCAAAACACGCAGTCGGTCCGGGGTGGCTGGAAGGACAGCCAAAGACTTCGTCCGGAAAGCTTTCCAGCAAGGTCTCATCTCTCAACAGCAGGCTAACCAGTGGCTTAACTCATAG
[0039] (a) Sequential features:
[0040] ●Length: 372bp, of which the effective length is 76-369bp
[0041] ●Type: base sequence
[0042] ●Chain type: single chain
[0043] ●Topological structure: linear
[0044] (b) Mo...
Embodiment 2
[0050] 1) Construction of the recombinant expression vector of Chinese prawn anti-lipopolysaccharide factor FcALF
[0051] Based on the sequence information of the mature peptide encoded by the cDNA sequence of ALF of Penaeus prawn (NCBI sequence registration number: AY859500) and the characteristics of the multiple cloning site of the secreted expression vector pPIC9k, primers were designed, and the upstream primer PRI-A1 contained an EcoR I restriction site ; Downstream primer PRI-A2 contains Not I restriction site.
[0052] PRI-A1:
[0053] GC GAATT CCAAGGGTGGGAGGCTGTGGCA (underlined is the EcoR I restriction site)
[0054] PRI-A2:
[0055] TA G+GGCCGC CTATGAGTTAAGCCACTGG (the underline is the Not I restriction site)
[0056] The PCR reaction system is 20ul, as follows:
[0057] Ex Taq buffer 2μl
[0058] dNTPs (10mM) 0.4μl
[0059] PRI-A1 (10μM) 0.4μl
[0060] PRI-A2 (10μM) 0.4μl
[0061] Ex Taq 0.2μl
[0062] 1 μl of blood cell cDNA of Penaeus chinensis
[0...
Embodiment 3
[0082] Example 3 Antibacterial Activity Identification of Recombinant Protein rFcALF
[0083] (1) Detection of inhibition zone by Oxford cup method
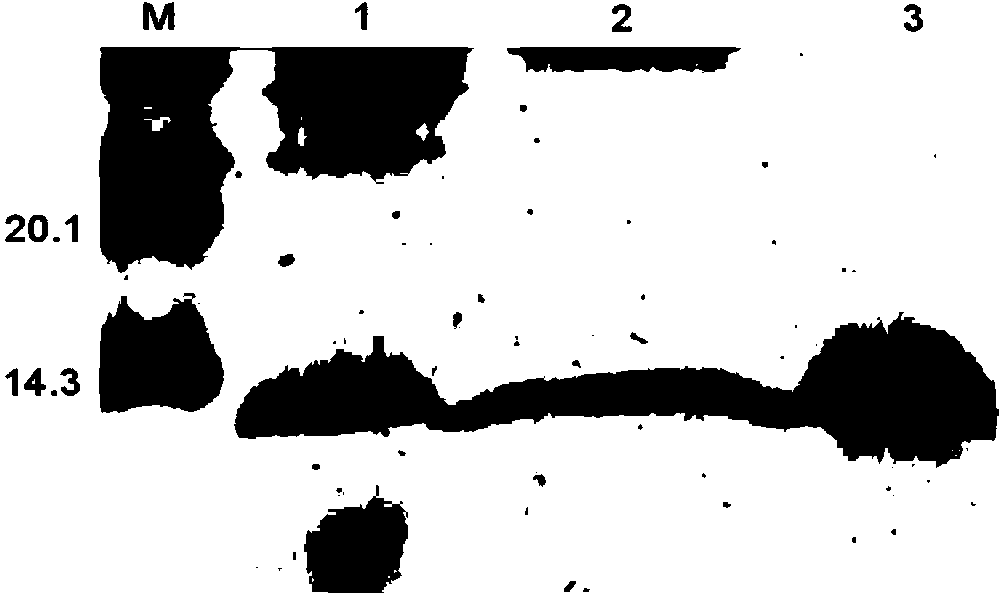
[0084] The cell concentration was 10 8 cell / mL of Escherichia coli (E.coli) and Staphylococcus aureus (Staphylococcus aureus) were uniformly coated on the LB solid plate, and placed in a 37°C incubator for 30-60min. After the surface of the plate was completely dry, place the Oxford cup, and then 200 μL of the above purified 50 mg / L recombinant protein solution was added thereto, and the same volume of 20 mM disodium hydrogen phosphate-sodium dihydrogen phosphate buffer solution at pH 7.2 was used as a negative control. Then place it in a 37°C incubator for 16-18 hours to observe the results, and then measure the size of the inhibition zone with a vernier caliper (see Figure 7 ), the results showed that 50mg / L recombinant FcALF could effectively inhibit the growth of Gram-negative bacteria E.coli.
[0085] (2) Use a microplat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com