Preparation method of pentaerythritol tetra(3-n-dodecylthiopropionate)
A technology of n-dodecyl thiopropionate and pentaerythritol tetra, which is applied in the field of preparation of sulfur-containing antioxidants, can solve the problems of great harm to the environment and operators, and difficult to achieve, so as to achieve reduced production costs, good quality, The effect of solving environmental pollution problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
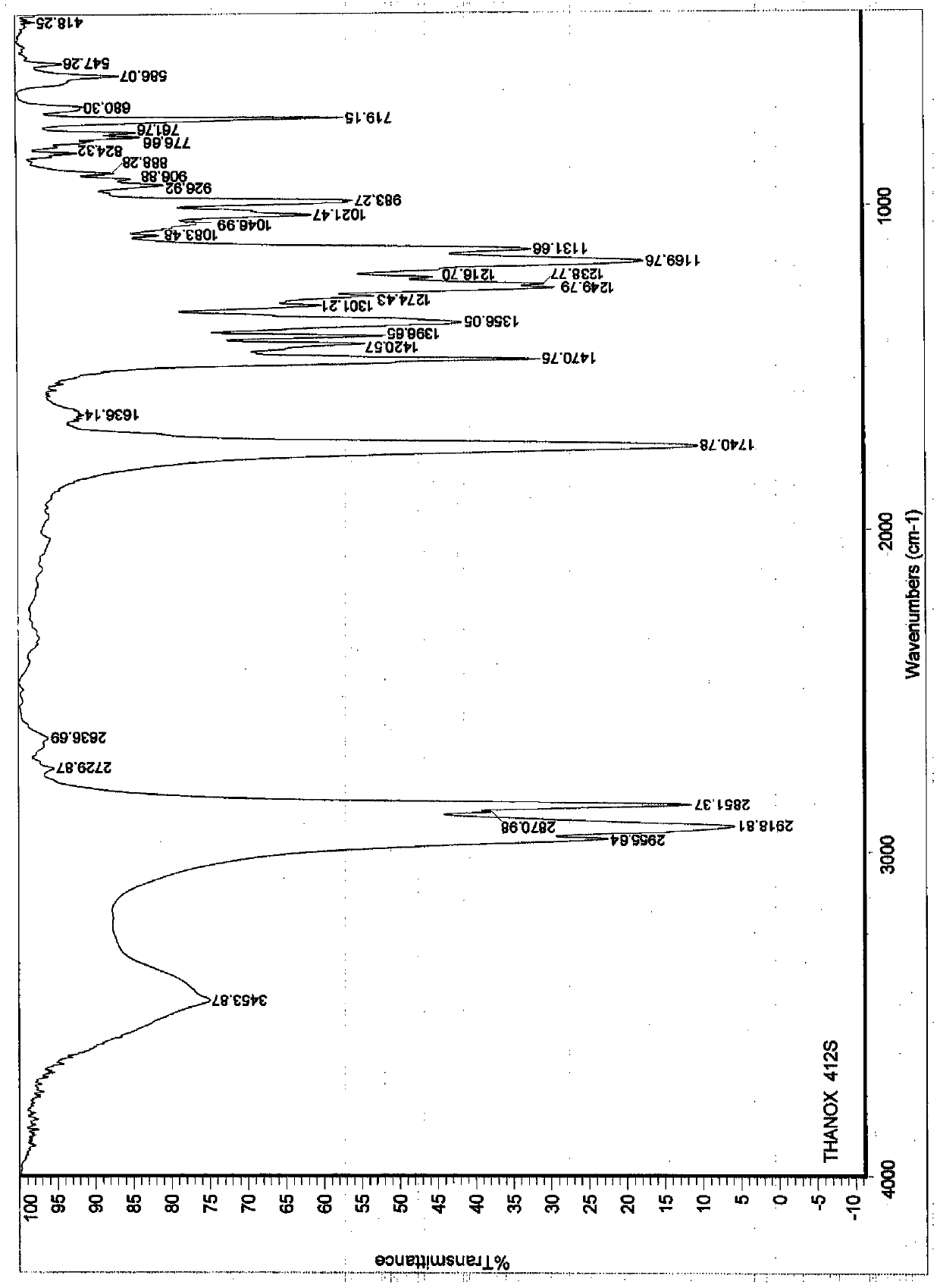
[0026] Add 161.9g (0.80mol) of 1-dodecylmercaptan (0.80mol) and 0.35g (0.0088mol) of sodium hydroxide to a 500ml reactor equipped with a stirrer, thermometer, condenser, nitrogen conduit and dropping funnel, and start to pass through Inject nitrogen, stir and heat up to 30°C, slowly add 70.4g (0.20mol) of pentaerythritol tetraacrylate dropwise to make the temperature between 30-45°C, then keep the reaction at 40°C for 4h, and recover excess 1-dodecyl by vacuum distillation Mercaptans. Adjust the pH to 6-7 with formic acid, recrystallize by adding methanol, filter and dry to obtain 217.4 g of white crystalline powder product, with a yield of 93.5% (based on pentaerythritol tetraacrylate). The obtained product has a liquid phase test purity of 99.1%, and the peak time is consistent with the standard sample, such as figure 1 As shown, the structure of the obtained product was characterized by infrared spectroscopy [2919cm-1 (methyl), 2851cm-1 (methylene), 1741cm-1 (ester carbony...
Embodiment 2
[0028] Add 166.0g (0.82mol) of 1-dodecylmercaptan and 0.35g (0.0065mol) of sodium methoxide into a 500ml reactor equipped with a stirrer, a thermometer, a condenser, a nitrogen conduit and a dropping funnel, and start feeding Nitrogen, stirred and heated to 30°C, slowly added 70.4g (0.20mol) of pentaerythritol tetraacrylate dropwise to make the temperature between 30-45°C, then kept at 40°C for 4 hours, and recovered excess 1-dodecylsulfur by distillation under reduced pressure alcohol. Adjust the pH to 6-7 with formic acid, add methanol for recrystallization, filter and dry to obtain 224.4 g of a white crystalline powder product with a yield of 96.6% and a purity of 99.2%.
Embodiment 3
[0030] Add 170.0g (0.84mol) of 1-dodecylmercaptan and 0.53g (0.0098mol) of sodium methoxide into a 500ml reactor equipped with a stirrer, thermometer, condenser, nitrogen conduit and dropping funnel, and start feeding Nitrogen, stirring and heating up to 40°C, slowly dropwise add 70.4g (0.20mol) of pentaerythritol tetraacrylate to make the temperature between 40-55°C, then keep the reaction at 50°C for 6h, and recover excess 1-dodecylsulfur by distillation under reduced pressure alcohol. Adjust the pH to 6-7 with glacial acetic acid, add absolute ethanol for recrystallization, filter and dry to obtain 226.2 g of a white crystalline powder product with a yield of 97.3% and a purity of 99.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com