Human kallikrein of mammalian cell expression as well as coding gene and application thereof
A technology of kallikrein and coding genes, which is applied in the field of trace detection of recombinant proteins, can solve the problems of protein molecular weight and charge difference, low protein biological activity, high-efficiency expression, etc., and achieve the effect of high accuracy and sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
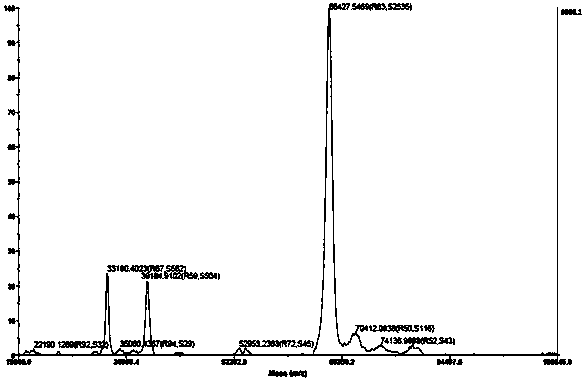
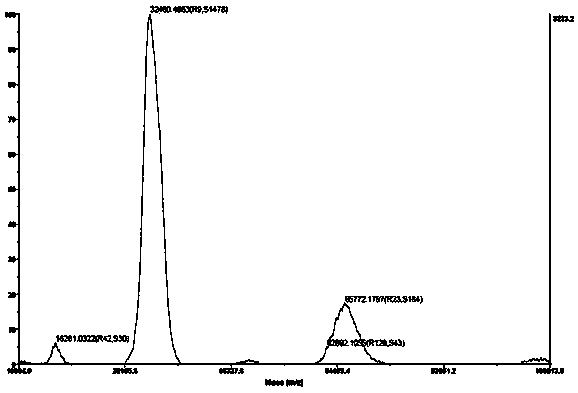
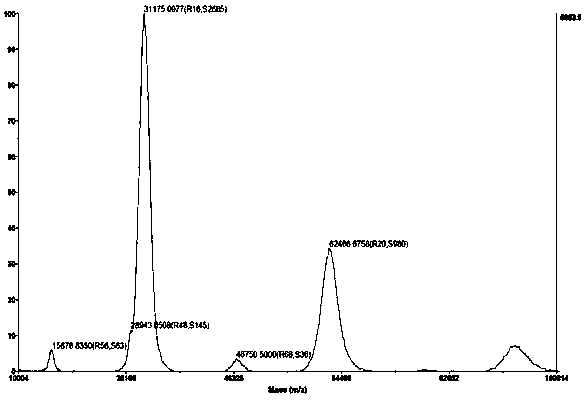
Image
Examples
Embodiment 1
[0082] Example 1 Recombinant hK1 gene optimization design
[0083] 1. Codon optimization
[0084] Combining the published cDNA sequence information of human kallikrein-1 sequence (Homo sapiens kallikrein1) in GenBank and the amino acid sequence of the product human kallikrein-1 that has been on the market, the cDNA sequence was determined (GenBank accession number: BC005313) (A gene without codon optimization, the original gene) and the published amino acid sequence (GenBank accession number: AAA59455.1) of human kallikrein 1 (Homo sapiens kallikrein) obtained the present invention after codon optimization of the gene The recombinant hK1 gene, as shown in SEQ ID No: 1.
[0085] The following is the codon optimization of the recombinant hK1. The parameters before and after optimization are compared as follows:
[0086] 1. Codon Adaptation Index (CAI)
[0087] Depend on Figure 2-a It can be seen that before the codon optimization, the codon adaptation index (CAI) of the r...
Embodiment 2
[0098] Example 2: Construction of recombinant hK1 gene expression vector in mammalian cells
[0099] 1. Construction of recombinant hK1 gene expression vector in adherent mammalian cell CHO-K1
[0100] The optimized recombinant hK1 (as shown in SEQ ID NO: 1) was directly fused to the fragment synthesized by the optimized signal peptide gene of Rattus norvegicus Anionic trypsin-1 protein (as shown in SEQ ID No: 3), and constructed into pUC57 plasmid (Provided by Nanjing GenScript Technology Co., Ltd.), a long-term storage plasmid was obtained, which was designated as pUC57-opt-hK1 plasmid. Using the pUC57-opt-hK1 plasmid as a template, the upstream primer P1 was introduced into HindIII and the downstream primer P2 was introduced into the BamHI restriction site for PCR amplification. The sequences of the primers used are as follows:
[0101] Upstream primer P1: CCCAAGCTTGCCACCATGTCCG
[0102] Downstream primer P2: CGCGGATCCTCAACTGTTTTCAGCAAT
[0103] The total reaction volu...
Embodiment 3
[0109] Example 3: Preparation of CHO host cells containing optimized recombinant hK1 gene
[0110] 1. Preparation of CHO-K1 adherent host cells containing optimized recombinant hK1 gene
[0111] Spread an appropriate amount of CHO-K1 (CCL-61, purchased from ATCC) cells in 24-well plates, and then add different concentrations of G418 (E859-5G, purchased from Amresco) containing 5% FBS (FSP500, purchased from Jitai) Biological) DMEM / F12 (MD207-050, purchased from Merck) medium. After 2 weeks, the MTT (0793-5G, purchased from Amresco) method determined that the working concentration of G418 was between 400-800ug / mL, and the working concentration of G418 in this application was 600μg / mL, see Figure 7-a .
[0112] After the correctly sequenced pCMV13-opt-hK1 plasmid was electrotransfected into CHO-K1 cells, G418 medium containing a concentration of 600 μg / mL was added and placed at 37°C, 5% CO 2 incubator culture. After 2 weeks, multiple cell clusters appeared under the micr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Relative molecular mass | aaaaa | aaaaa |
| Relative molecular mass | aaaaa | aaaaa |
| Relative molecular mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com